2- Innate Immunity: Immediate Response
1/127
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
128 Terms
What is the 1st line of immunity?
prevents mo getting in, barriers & molecular mechanisms
Barriers
biggest is skin, epithelial & endothelial cells of BV
ex → skin, mucosal surfaces, secretions (tears, sebum, sweat)
What is the 2nd line of immunity?
induced intrinsic mechanisms
ex → pattern-recognition receptors, bind to pathogen-associated molecular patterns (PAMPs)
What is the 3rd line of immunity?
adaptive immunity, only if innate was overrun or not successful
some mechanisms of innate still going on
When does the 2nd line of defense get activated?
mobilized once cells of immune system detects presence of infection
How are innate immune proteins made?
gene expression changes in certain subset of cells = proteins
What are mechanical barriers?
prevent access
epithelial cells joint by tight junctions
skin & gut → air/fluid flow to expel mo, peristalsis in gut
lungs → mucociliary escalator, movement of mucus by cilia to move up & out, cough up, trap & target bacteria
eve/nose/oral cavity → tears, nasal cilia
What mechanical barrier does the skin have?
epithelial cells joined by tight junctions
longitudinal flow of air or fluid
What chemical barriers does the skin have?
fatty acids → sebum
antimicrobial peptides → disrupt microbial membrane
What microbiological barrier does the skin, gut, lungs, ENT have?
normal microbiota
What mechanical barrier does the gut have?
epithelial cells joined by tight junctions
longitudinal flow of air or fluid
peristalsis in gut
What chemical barriers does the gut have?
low pH
antimicrobial enzymes → lysozyme, disrupt cell wall of bacteria
antimicrobial peptides
What mechanical barriers do the lungs have?
epithelial cells joined by tight junctions
mucociliary escalator
movement of mucus by cilia to move up & out
cough up, trap & target bacteria
What chemical barriers do the lungs have?
pulmonary surfactant
antimicrobial peptides
What mechanical barriers do the eyes/nose/oral cavity have?
epithelial cells joined by tight junctions
tears, nasal cilia
What chemical barriers do the eyes/nose/oral cavity have?
antimicrobial enzymes → tears & saliva
antimicrobial peptides
Antimicrobial Peptides & Mucus
anywhere there’s mucus, will be antimicrobial peptides secreted into mucus
most bind to & disrupt membranes
Lysozyme
targets & disrupts cell wall of bacteria
Where do we want/not want microbiota to be?
mucosal surfaces, tell foreign ones to get out
don’t want microbes on mucosal surfaces of lungs
How does the immune system organize itself?
launches multiple plans, never want plan A to fail, other wise too late for plan B
Microbiota
commensal relationship
nutrition
help w/ metabolism, use theirs to break thing down
general health (keep pathogens in check)
Cells in Human Body
3 × 10^13 human cells
3.8 × 10^13 bacterial cells, more bacteria than human cells
>1000 species of bacteria in gut
move down GI tract = bacteria increases/concentrates → due to peristalsis
mainly from mom, given in pregnancy & vaginal birth
Can the innate immune response stop?
No, once it starts won’t stop
continues even after subsequent steps
continues throughout battling pathogen
What does the immune system recognize?
intracellular & extracellular pathogens
Extracellular Pathogens
grow outside of cells → replicate in space in human cells
bacteria, viruses, parasites, fungi
viruses recognized extracellularly during movement w/in body not during replication
fungi only extracellular
brought in → phagosome/phagolysosome do endocytosis, engulf & bring in w/ vesicle
still extracellular → top equiv EC & vesicle
replicate outside
viruses not extracellular pathogen, sometides exist otuside of/traveling to or in host cells
Intracellular Pathogens
replicate & live inside cells
viruses, intracell bacteria & pathogens,
viruses replicate & assemble new virions intracellularly
pathogen can live otuside or in vesicle = extracell still
What replicates inside macrophages?
mycobacteria
Why are viruses never extracellular pathogens?
must be inside living cell to replicate to be an extracellular pathogen
What is the complement pathway?
.series of protein in blood working to enhance immune response
lead to destroying pathogens → opsonization, MAC forming
What are the 3 ways of complement activation?
alternative, lectin, clasical
waterfall → get to certain point, going & can’t be stopped
3 streams converging on waterfall
Which proteins are involved in the complement pathway?
proteins C1-C9
Alternative Pathway
1st complement pathway to be activated, start of infection, happening in proximity to microbe, spontaneous
Lectin Pathway
2nd to be activated, binds to sugars on microbes
Classical Pathway
last to be activated, needs antibodies to be activated
induced, antibodies bound to microbe’s surface
What do lectin & classical pathways share?
started by specific binding events, binding of receptors to microbes
How long do antibodies take to be made?
2 weeks
What do the 3 complement pathways converge on?
C3 protein, have 3 outcomes/effector functions
C3 always cleabed into C3a & b
fixed when C3b attaches to surface of protein/bacteria
Which pathway involves C3 & C5?
recruiting inflammatory cells to clear microbe/phagocytes
Which pathway is more unique for C3?
opsonizing pathogens, facilitating uptake & killing by phagocytosis
What is mediated by C5?
perforation of pathogen cell membranes
make complex poking holes in PM, induce lysis
Which proteins are exclusive to lectin & classical?
C1, 2, 4
T/F: phagocytosis & perforation aren’t either/or effector functions
True, processes start at same time
Where do pathways culminate?
in elimination of infecting pathogen
How many proteins are involved in the complement paths?
9, C1-9
What is the alternative complement pathway?
A part of the immune system that enhances the ability to clear pathogens from an organism, activating complement proteins without antibody involvement and promoting opsonization and cell lysis.
serum proteins in blood, not only active against blood infections
go out into tissue w/ vasodilation from inflammatory response
circulating in blood
functionally inactive
initially gets triggered & cleaved, become active
What does an infection trigger?
complement activation
cascade of enzymatic react ions
each protease cleaves & activates next protease, pull e/o trigger
What is upstream of C3 in alternative pathway?
C1, C2, C4
What is the general process of alternative pathway?
C3 in bloodstream
C3 interacts w/ H2O in bloodstream to form iC3
iC3 recruits factor B → makes iC3B
iC3B complex recruits factor D to cleave factor B & form iC3Bb
iC3Bb recruits 2nd C3 floating around in blood, cleaves C3 into C3a & C3b
C3b does 3 things: opsonize, inflammation, pores in membrane
How does C3 interact with H2O?
hydrolyzed in bloodstream
forms iC3 (induced C3)
undergoes conformational change
happens in absence/presence of infection, random bacteria or infection
How does iC3 recruit factor B?
iC3 has a binding site for factor B that allows factor B to attach and initiate the cleavage process by factor D, forming iC3B
What happens when iC3B recruits factor D?
Factor D cleaves factor B into Ba and Bb.
B more susceptible to proteases when bound to iC3, gets cleaved by factor D
Ba released, Bb stays w/ iC3 = iC3Bb = C3 convertase
What happens whe iC3Bb recruits a 2nd C3?
C3 cleaved into C3a & C3b
C3b covalently binds to pathogen surface
C3a diffuses away, causes inflammation, recruits immune cells
What is the only thing bound to pathogen surface?
C3b, only thing deposited on cell surface
What acts as an opsonizer?
C3b, receptors bind to it & it acts as opsonizer/eat me signal → induces phagocytosis
How does C3b make a C3 convertase?
C3b binds Bb to become C3bBb = C3 convertase
Factor B & D implied
C3bBb has same function as iC3Bb but can make easier, prefer C3bBb
C3Bb can cleave C3 to C3a & C3b
How does C3b make a C5 convertase?
C3b binds Bb & another C3b to make C3b2Bb/C5 convertase
C3b2Bb cleaves C5 → C5a & C5b
Why does C3Bb cleave C3 to C3a & C3b?
want a lot of opsonizing tags on pathogen (C3a & need C3b to make lots of pores in membrane
Why does C3b2Bb cleave C5 to C5a & C5b?
C5a → diffuses away & causes inflammation
C5b → stays w/ pathogen, not connected to membrane, used for further MAC formation
What initiates formation of the MAC?
C5b, a reason why C5 convertase is needed for optimal pathogen elimination
B cells develop in the ____ & T cells develop in the ____
bone marrow, thymus
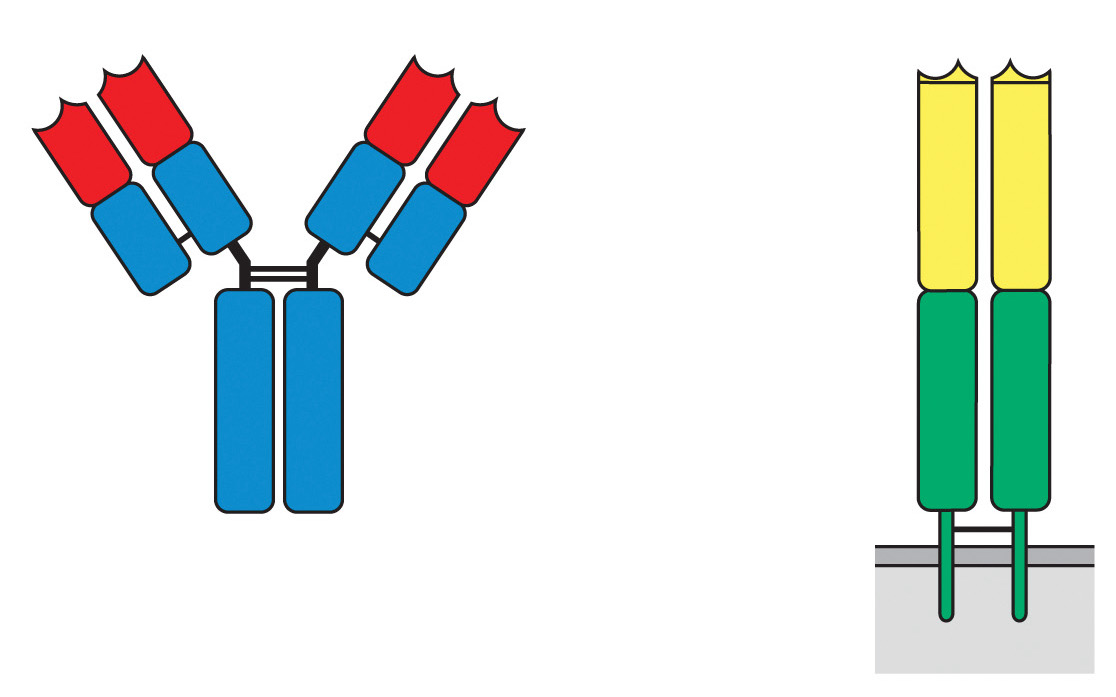
Which region of the antibody & the TCR is variable?
red, yellow
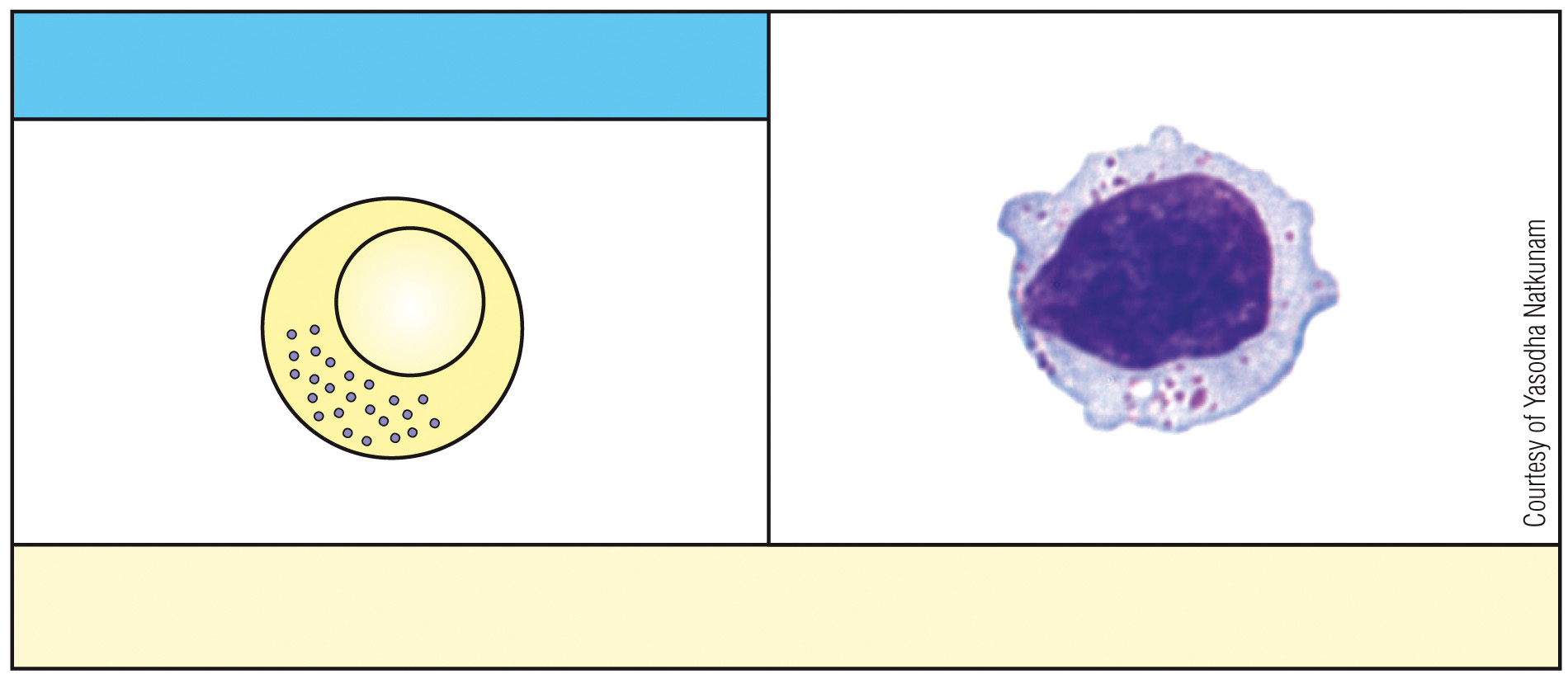
What is this cell & what is its function?
NK cell, kills virus-infected cells
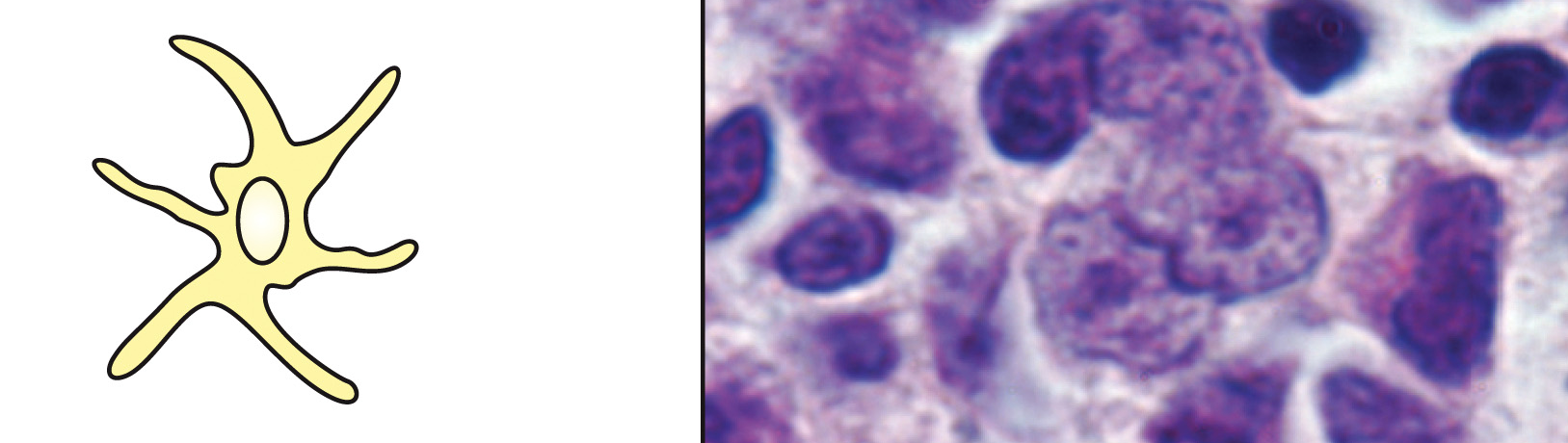
What is this cell & what is its function?
dendritic cell, phagocytosis
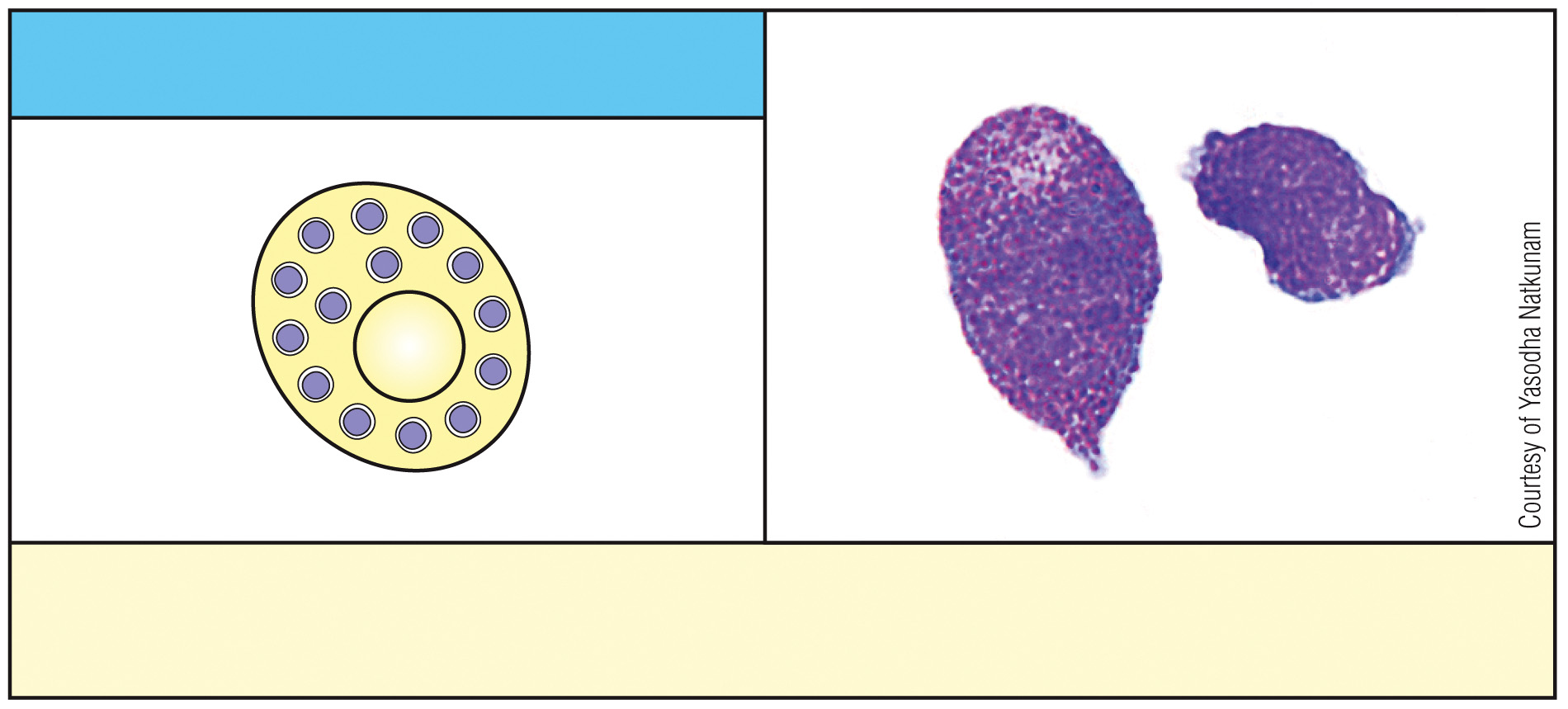
What is this cell & what is its function?
mast cell, expulsion of parasites
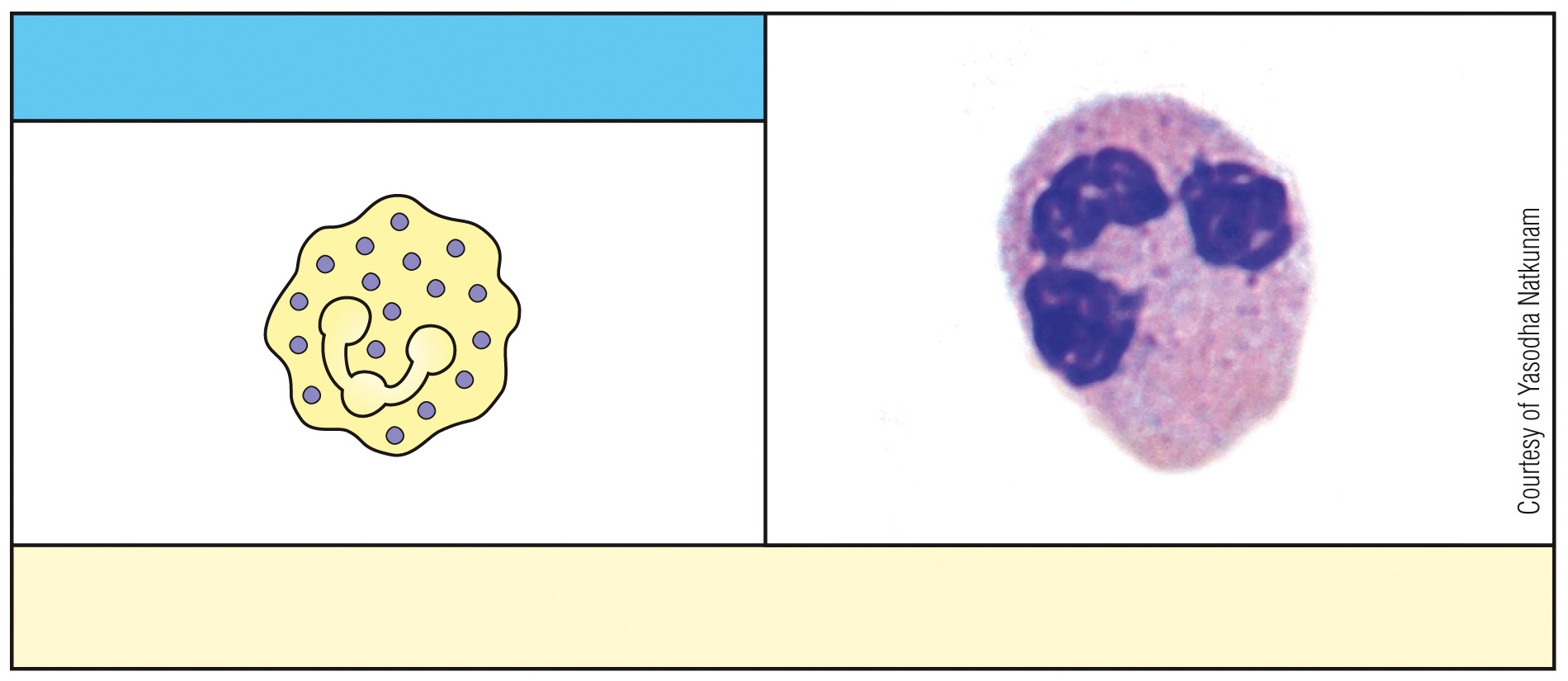
What is this cell & what is its function?
neutrophil, phagocytosis
Which of the following is NOT a primary lymphoid organ?
A) Spleen
B) Lymph node
C) Lymph vessels
D) Thymus
A/B/C (spleen, lymph node, lymph vessels) are secondary lymphoid organs
How is the MAC complex formed?
C5b made from C5 convertase/C3b2Bb cleaving C5
C5b binds C6
C5b & C6 recruit C7, C7 binds to membrane, peripheral protein
C8 recruited, inserts into pathogen membrane
C9 recruited & oligomerizes (many C9s), make pore in pathogen membrane
When C5 binds to C6, are they membrane bound?
no, only become membrane-bound when C7 & C8 bind to the complex
Which protein is transmembrane in the alternative complement pathway?
C8 & C9
Which protein is peripheral in the alternative complement pathway?
C5b & C6
What proteins make up the MAC?
C5b, C6, C7, C8, C9
What are the microbe targets of complement pathways?
bacteria, fungi, parasites, archaea, viruses
T/F: Archaea are microbial targets of complement pathways & infect us.
False, are targeted but don’t infect us
T/F: Viruses are always microbial targets of complement pathways.
False, only if they have a membrane, can’t target non-enveloped viruses.
Classical Pathway
C1q binding to antibody on surface, leads to breakdown of C2 & C4
Lectin Pathway
mannose-binding lectin (MBL) bids to carb, leads to breakdown C2 & C4
Where do classical & lectin paths converge?
converge at formation of C4b2a, C3 convertase, diff version of iC3Bb
What are complement regulatory proteins?
regulate & protect host cells from complement activation, they prevent damage to host tissues by inhibiting complement pathways.
Soluble Factors - CRP
complement regulatory proteins, secreted in bloodstream, activate or inhibit complement
Membrane Bound Factors - CRP
complement regulatory protein, don’t eat me signals on our cells, inhibit complement
Properdin
complement regulatory protein, soluble factor activating complement
binds C3bBb/C3 convertase, stabilizes it
activate C3bBb, keeps breaking C3 → C3a&b
lock in place to work a bunch
What is the function of factor H & I?
soluble factors that inactivate complement
C3b on surface
Factor H binds C3b
Factor H recruits factor I
Factor I cleaves C3b into iC3b(inactivated)
What do factor H & I do in an active infection?
no factor H or I to inhibit complement
Where should the complement pathway not occur?
health host cells & tissues, prevent damage
gut especially → don’t want inflammation
microbiota already there
What happens if the complement occurs in places where the microbiota is?
inflammation, IBS/autoimmune, want to inhibit to stop/limit inflammation
Where are DAF & MCP?
membrane bound on surface of OUR cells
What is the function of DAF & MCP?
inhibit complement early, prevent actively killing out ells
DAF binds C3bBb & displaces Bb
mCAP binds C3bBb & displaces Bb
How does DAF work?
binds C3bBb & displaces Bb, unlikely that phagocyte will find C3b leftover before degraded by ells
disrupt C3 covnertase
How does MCP work?
binds C3bBb, displacing Bb and preventing complement activation on our cells.
MCP-C3b recruits factor I, shove out & bring in/cleave C3b
factor I cleaves C3b to iC3b/inactivates, prevents acting as opsonin, X attack healthy cells
How does MAC work?
destruction of target cells occurs w/ influx of water & loss of electrolytes
What happens if DAF/MCP don’t do their job?
Complement activation leads to uncontrolled inflammatory response, causing damage to healthy cells.
Why do we need DAF/MCP?
extra measures to save our cells if complement isn’t stopped at C3 level
don’t want inflammatory death/lysis of our cells
if need to kill → want to induce apoptosis
What prevents recruitment of C9?
CD59, binds to C5b678, no pore formation
T/F: MAC formation can be stopped at C5.
False, can’t stop initiation of MAC from C5
How does opsonization occur?
C3b on pathogen binds CRI (complement receptor 1) on phagocyte
interacts w/ complement receptors on macrophage, induce phagocytosis
macrophage forms phagosome
macrophage membranes fuse to create phagosome
lysosomes fuse w/ phagosome to form a phagolysosome, destroy microbe
What leads to phagocytosis?
C3b on pathogen engaging/binding w/ CR1, initiates signaling that leads to phagocytosis & breakdown of microbe
How do macrophages interact w/ pathogen’s bound C3b?
macrophages have receptors for C3b fragments, complement receptor CR1/3/4, bacteria w/ C3b coat = better phagocytosis = opsonization
What are anaphylotoxins?
promote inflammation, C3a & C5a
recruit immune cells in bloodstream
increase vascular permeability
How do anaphylotoxins increase vascular permeability?
break tight junctions between endothelial cells
smaller things exit BV 1st (ex → complement, send out/enhance C3/5
cells can exit layer
positive feedback loop
Is C3a or C5a more potent?
C5a more potent than C3a, later in pathway committing to finishing pathway, start w/ less in case complement needs to be stopped