ALKENES AND ALKYNES
1/106
Earn XP
Description and Tags
2ND SHIFTING - ORGCHEM LEC
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
Unsaturated hydrocarbon
hydrocarbon (HC) molecule in which one or more
carbon– carbon multiple bonds (double bonds, triple bonds, or both) are present.
Alkenes
has one or more C—C double bonds.
Alkynes
has one or more C—C triple bonds.
Aromatic HC
has a special type of “delocalized” bonding.
Alkenes and Alkynes
follows similar reaction mechanism
Aromatic HC
follows different reaction mechanism from Alkenes and Alkynes
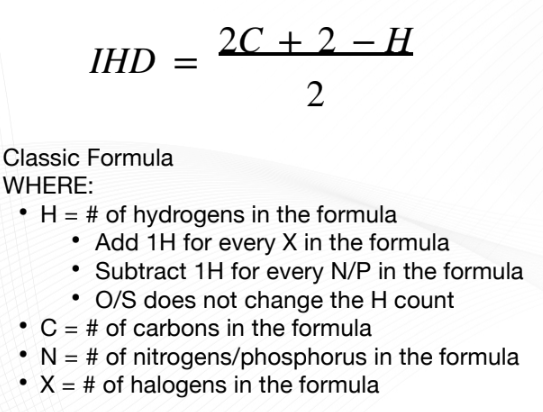
Index of Hydrogen Deficiency (IHD)
“number of sites of unsaturation”
number of pairs of hydrogen atoms that must be removed from
the corresponding “saturated” formula to produce the molecular
formula of the compound of interest.
result from cyclic structures and the presence
of multiple bonds (double/triple bonds).
computed from compounds with C, N, H, O, S, and X.
Classic Formula of IHD
Unified Formula of IHD
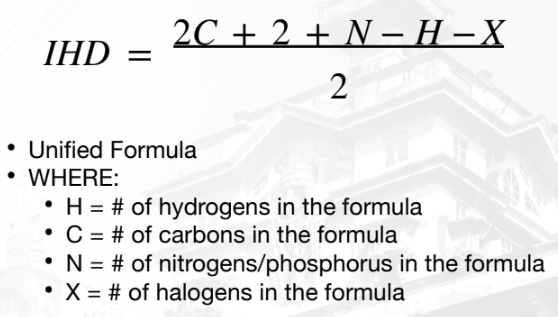
CnH2n
General Formula for Alkene
CnH2n-2
General Formula for Cycloalkene
2, 4
Alkenes have __ fewer -H atoms and cycloalkenes have _ fewer -H
atoms compared to alkanes with the same # of C’s.
-alkenyl
Alkene as substituent is called
Alkenes
commonly found in pheromones (sex attractant) and terpenes (aliphatic volatile oil)
gas
C2-C4 alkenes
liquid
C5-C17 alkenes with 1 C=C
solid at RT
more than C17 alkenes
CnH2n-2
General Formula for Alkyne
CnH2n-4
General Formula for Cycloalkyne
4
Alkynes have __ fewer hydrogens compared to alkanes with the same #
of C’s; occurs less commonly compared to alkenes.
closely similar; 2 pi bonds
The reactions of alkynes are ____ to that of the alkenes and
focuses on the carbons participating in the triple bond.
However, alkynes can accommodate one more addition reaction
because it possess _____ in the functional group.
polar mechanism; electrophilic addition reaction
Alkenes/Alkynes follows a ____ on its reaction, specifically
an ______.
less; more
The pi electrons are moved to the ___ stable
carbon and the ___ stable carbon
becomes an electrophile.
1
Only the more stable carbon in the double
bond will be an electrophile, and only __
reaction product will be formed.
Regiospecific reaction
when only ONE of the two addition product orientation
if formed.
follows the Markonikov’s Rule.
Markonikov’s Rule
proposed by Vladimir Markonikov after observing the addition of HX to an alkene.
“that in the addition reaction of alkenes, the more highly
substituted carbocation is formed rather than the less highly substitute
one”.
more stable carbocation is formed over the
less stable one.
more stable
When a pi bond is cleaved, the _____
carbon becomes a carbocation (C+).
adds
In the presence of other reagents that can serve as
nucleophile (Nü), the nucleophilic portion of the
reagent ___ to the carbocation and forms a new bond.
Hydration
Electrophilic Addition Reactions (AE) of Alkenes:
addition of water (H2O) to alkenes
destructive to molecules
H3PO4, HCl, H2SO4, and H+
Catalyst of Hydration
nucleophile (-OH); 2° ROH product
The _____ from H2O is added to the
carbocation and forms a ____.
hydroboration
Hydration reaction follows Markonikov’s Rule, except ____.
acid
STEPS IN HYDRATION:
pi-bond reacts with the (a=?) catalyst, providing the
H+. This result into bond cleavage and formation of
carbocation intermediate.
H2O acts as a nucleophile and adds itself to the
carbocation electrophile. This forms a (b=?).
The electrons between H and OH in the protonated
alcohol will (c=?) back to the O atom.
This will release the proton, regenerating the H+ taken
from the acid catalyst and forming the final product, a
(d=?).
a=
protonated alcohol
STEPS IN HYDRATION:
pi-bond reacts with the (a=?) catalyst, providing the
H+. This result into bond cleavage and formation of
carbocation intermediate.
H2O acts as a nucleophile and adds itself to the
carbocation electrophile. This forms a (b=?).
The electrons between H and OH in the protonated
alcohol will (c=?) back to the O atom.
This will release the proton, regenerating the H+ taken
from the acid catalyst and forming the final product, a
(d=?).
b=
delocalize
STEPS IN HYDRATION:
pi-bond reacts with the (a=?) catalyst, providing the
H+. This result into bond cleavage and formation of
carbocation intermediate.
H2O acts as a nucleophile and adds itself to the
carbocation electrophile. This forms a (b=?).
The electrons between H and OH in the protonated
alcohol will (c=?) back to the O atom.
This will release the proton, regenerating the H+ taken
from the acid catalyst and forming the final product, a
(d=?).
c=
2◦ alcohol
STEPS IN HYDRATION:
pi-bond reacts with the (a=?) catalyst, providing the
H+. This result into bond cleavage and formation of
carbocation intermediate.
H2O acts as a nucleophile and adds itself to the
carbocation electrophile. This forms a (b=?).
The electrons between H and OH in the protonated
alcohol will (c=?) back to the O atom.
This will release the proton, regenerating the H+ taken
from the acid catalyst and forming the final product, a
(d=?).
d=
BH3 / THF
H2O2, NaOH, H2O
Catalyst of Hydroboration
1. Hg(OAc)2, H2O
2. NaBH4
Catalyst of Oxymercuration-Demercuration
Fumarase
Catalyst of Enzymatic Hydration
Ether Formation
Electrophilic Addition Reactions (AE) of Alkenes:
addition of alcohol (ROH) to alkene
follows Markonikov’s rule to determine the more stable
carbocation to be formed.
H+ (acid)
Catalyst of Ether Formation
(RO-)
Ether Formation:
The nucleophile __ from ROH is added to the more stable
carbocation (C+).
H
STEPS IN ETHER FORMATION:
The double bond (C=C) electrons abstracts the (a=?) from the acid catalyst or even from the RO—H itself, breaking the double bond and producing a carbocation.
Subsequently, the carbocation is then attacked by the nucleophile (b=?) group attaching itself to the stable carbocation and forming an (c=?) product.
a=
(RO-)
STEPS IN ETHER FORMATION:
The double bond (C=C) electrons abstracts the (a=?) from the acid catalyst or even from the RO—H itself, breaking the double bond and producing a carbocation.
Subsequently, the carbocation is then attacked by the nucleophile (b=?) group attaching itself to the stable carbocation and forming an (c=?) product.
b=
ether
STEPS IN ETHER FORMATION:
The double bond (C=C) electrons abstracts the (a=?) from the acid catalyst or even from the RO—H itself, breaking the double bond and producing a carbocation.
Subsequently, the carbocation is then attacked by the nucleophile (b=?) group attaching itself to the stable carbocation and forming an (c=?) product.
c=
Hydrohalogenation
Electrophilic Addition Reactions (AE) of Alkenes:
addition of hydrogen halide (HX) to alkenes.
follows Markonikov’s Rule, where the nucleophilic (X-) is added to the more stable carbocation in an ether catalyzed solution.
monohalogenated alkane
Product of Hydrohalogenation
ether
Catalyst of Hydrohalogenation
nucleophile (X-) from hydrogen halides (HF, HCl, HBr, or HI)
added to alkenes to form monohalogenated alkane as a product in Hydrohalogenation
Halogenation
Electrophilic Addition Reactions (AE) of Alkenes:
follows the same mechanism as HX and H2O addition, with the
exception of Br2 addition occurring with anti-stereochemistry (trans-).
nucleophile (X-) from single mole of halogen (Cl2 or Br2)
added to alkenes in Halogenation
DCM/CCl4 catalyst
catalyst of halogenation
dihalogenated alkanes
product of halogenation
anti-stereochemistry (trans-)
orientation of halogenation
Halohydrin Formation
Electrophilic Addition Reactions (AE) of Alkenes:
addition of halogen (X2) in H2O to alkenes
follow Markonikov’s rule
nucleophile of -X and -OH
added to alkene in halohydrin formation
halohydrin product
product of halohydrin formation
anti-stereochemistry (trans-)
orientation of halohydrin formation
Hydrogenation/Reduction
Electrophilic Addition Reactions (AE) of Alkenes:
addition of hydrogen (H2) to alkenes.
double bond is reduced to a single bond.
H2 atom
added to alkenes in Hydrogenation
Pd/PtO2
catalyst of Hydrogenation
saturated products
product of Hydrogenation
syn stereochemistry (cis-).
orientation of hydrogenation
Epoxidation
Electrophilic Addition Reactions (AE) of Alkenes:
addition of peracid (RCOOOH) to alkene
Peracid/Peroxy Acid
class of organic compound with a -OOH group.
(RCOOOH)
m-chloroperoxybenzoic acid (mCPBA)
compound classified as a
peroxy acid and a common reagent used for epoxidation
epoxide → vicinal diol
product of epoxidation
Oxidation
Electrophilic Addition Reactions (AE) of Alkenes:
exposure of alkene to an oxidizing agent (e.g., KMnO4)
Hydroxylation
mild oxidation
KMnO4 in basic solution (H2O/NaOH)
catalyst of Hydroxylation
vicinal diol
product of Hydroxylation
syn stereochemistry (cis-)
orientation of Hydroxylation
Bond cleavage
strong oxidation
oxidizing agent (KMnO4) in acidic solution (H3O+).
catalyst of Bond Cleavage
ketone
2 oxidized bonds = ?
RCOOH
3 oxidized bonds = ?
CO2
4 oxidized bonds = ?
Ozonolysis
Electrophilic Addition Reactions (AE) of Alkenes:
oxidation reaction of alkene using ozone (O3).
oxidative cleavage of the C-C multiple bonds (double/triple
bonds) among alkenes/alkynes.
O3 with a metal catalysts Zn
catalyst of Ozonolysis
carbonyl products with the more stable carbocation
product of Ozonolysis
Polymerization
Electrophilic Addition Reactions (AE) of Alkenes:
addition of a radical to alkenes.
radical adds up to the alkene to undergo propagation of the
polymer.
The newly formed radical repeats the radicalization
process as many times to elongate the polymer.
Benzyloxy radical (BzO•).
added to alkene in Polymerization
longer carbon chains with repeating polymers
product of Polymerization
Hydrogenation
Electrophilic Addition Reactions (AE) of Alkynes:
addition of hydrogen (H2) to alkynes.
Pd/C or Lindlar’s Catalyst
catalysts of Hydrogenation in Alkynes
H2
added to alkynes in Hydrogenation
syn stereochemistry (cis-).
orientation of Hydrogenation in Lindlar’s catalyst in Alkynes
Hydrohalogenation
Electrophilic Addition Reactions (AE) of Alkynes:
addition of hydrogen halide (HX) to alkynes.
follows Markonikov’s Rule, where the nucleophilic (X-) is added to the more stable carbocation formed.
HX
added to alkynes in Hydrohalogenation
monohalogenated alkenes.
product of Hydrohalogenation in Alkynes
geminal halides or dihalide alkanes
HYDROHALOGENATION:
With excess HX, alkynes can undergo further reaction forming _____.
Halogenation
Electrophilic Addition Reactions (AE) of Alkynes:
addition of halides (X2) to alkynes.
follows the same mechanism with X2 and alkenes
X2
added to alkynes in Halogenation
vicinal halide
product of Halogenation in Alkynes
tetrasubstituted alkyl halide
HALOGENATION:
With excess X2, the reaction can result into the formation of
_______.
anti-stereochemistry (trans-) product
orientation of Halogenation in Alkynes
Hydration
Electrophilic Addition Reactions (AE) of Alkynes:
addition of water (H2O) to alkynes
H2O
added to Alkynes in Hydration
H2SO4/HgSO4
catalyst of Hydration in Alkynes
enol (alkene alcohol)
intermediate product of Hydration in Alkynes