1. Basic Atomic Structure
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
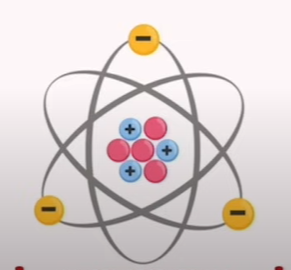
🧪 Table: Parts of an Atom
Component | Description |
|---|---|
Protons | A stable subatomic particle in the atomic nuclei with a positive charge equal in magnitude to that of an electron, but of opposite sign. |
Neutrons | A subatomic particle of about the same mass as a proton but without an electrical charge. |
Electrons | A negatively charged subatomic particle that is 1/1836 times smaller than a proton. |
Electric Charge | - Neutrons = Neutral - Protons = Positive - Electrons = Negative |
Important Note | The number of protons and electrons is equal in a neutral atom. |
❓ Flashcard Questions (Numbered)
What subatomic particle has a positive charge and is found in the atomic nucleus?
Which subatomic particle has nearly the same mass as a proton but no charge?
Which subatomic particle is much smaller than a proton and carries a negative charge?
What does it mean when a neutron is called "neutral"?
How much smaller is an electron compared to a proton?
What is the relationship between protons and electrons in a neutral atom?
Which particle has a charge equal in magnitude to an electron but opposite in sign?
What can you infer about the charge of an atom if it has more electrons than protons?
Proton
Neutron
Electron
It has no electrical charge
1/1836 times smaller
They are equal in number
Proton
The atom would have a negative charge
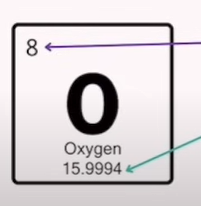
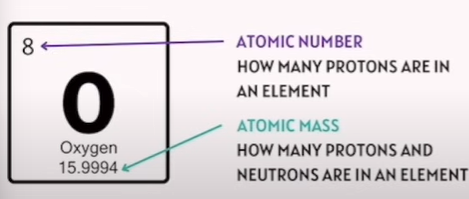
Explain what each number mean in this screenshot
Component | Mass | Mass Unit | Notes |
|---|---|---|---|
Proton | 1.67 × 10⁻²⁴ g | 1 amu | Found using atomic number |
Neutron | 1.67 × 10⁻²⁴ g | 1 amu | Mass similar to proton |
Electron | 9.109 × 10⁻²⁸ g | 0.000549 amu | Not counted in atomic mass |
Atomic Number | Number of protons in an element | — | Also equals number of electrons in a neutral atom |
Atomic Mass | Number of protons + neutrons in an element | — | Used to calculate neutron number |
Neutron Formula | Neutrons = Mass Number − Atomic Number | — | Example: Oxygen has 8 protons and 8 neutrons |
Mnemonic: PAN MAN | - P = Protons - A = Atomic Number - N = Number of Protons - M = Mass Number - A = Add - N = Neutrons | — | Helps memorize relationships between protons, mass, and neutrons |
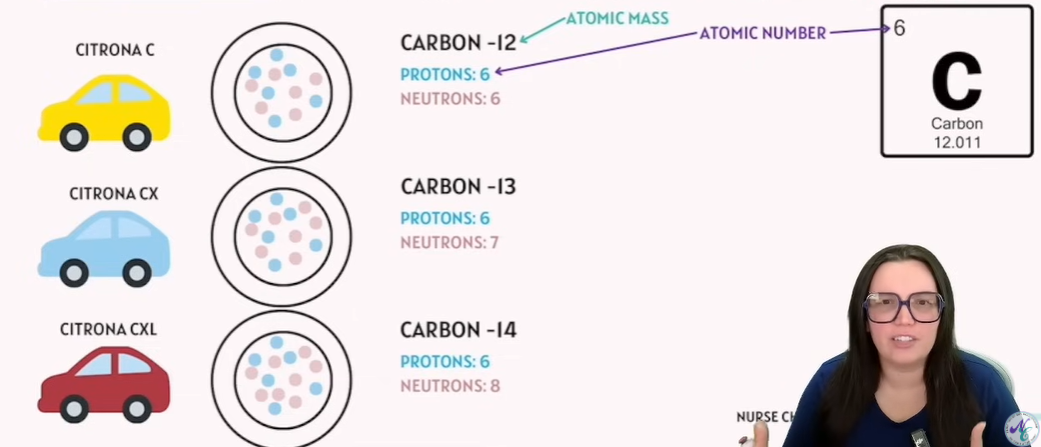
What does amu stands for?
AMU stands for atomic mass unit.
What is Isotopes
Distinct, Nuclear species of the same chemical element.
They have the same atomic number and position in the periodic table.
They have the same number of protons but different numbers of neutrons.
❓ TEAS-Style Multiple Choice Questions (Isotopes & Atomic Structure)
Which of the following best defines an isotope?
A. Atoms with different numbers of protons and electrons
B. Atoms with the same number of protons but different numbers of neutrons
C. Atoms with different numbers of protons and neutrons
D. Atoms with the same mass number but different atomic numbers
If two atoms are isotopes of the same element, what must be true?
A. They have the same number of neutrons
B. They have the same number of protons
C. They have different atomic numbers
D. They have different numbers of electrons
Which subatomic particle determines the identity of an element?
A. Neutron
B. Electron
C. Proton
D. Mass number
What is the mass number of an atom with 11 protons and 12 neutrons?
A. 12
B. 11
C. 23
D. 1
Why do isotopes of an element have nearly identical chemical behavior?
A. They have the same number of neutrons
B. They have the same atomic mass
C. They have the same number of electrons
D. They have the same number of nucleons
✅ Answer Table (Correct Answers Only)
Question | Correct Answer | Choice Text |
|---|---|---|
1 | B | Atoms with the same number of protons but different numbers of neutrons |
2 | B | They have the same number of protons |
3 | C | Proton |
4 | C | 23 |
5 | C | They have the same number of electrons |
❓ TEAS-Style Multiple Choice Questions (Ions and Cations)
What is an ion?
A. An atom with no charge
B. An atom or group of atoms with a net electrical charge
C. An atom with an equal number of protons and electrons
D. An atom that always has a positive charge
How does an atom become a cation?
A. By gaining electrons
B. By losing protons
C. By losing electrons
D. By gaining neutrons
Which of the following describes a cation?
A. A negatively charged ion
B. A neutral atom
C. A positively charged ion
D. An atom with more electrons than protons
What charge does a sodium cation (Na⁺) have?
A. -1
B. +1
C. 0
D. +2
CATION VS ANION
Term | Definition |
|---|---|
Ion | An atom or a group of atoms with a net electrical charge. An atom becomes an ion if it gains or loses electrons. |
Cation | A positively charged ion. Formed when an atom loses electrons. |
Example: Sodium (Na)
State | Protons (+) | Electrons (−) | Charge |
|---|---|---|---|
Neutral Sodium (Na) | 11 | 11 | No charge (neutral) |
Sodium Cation (Na⁺) | 11 | 10 | +1 charge (cation) |
Sodium atom loses 1 electron → becomes Na⁺ (cation).
Protons stay the same (11), electrons decrease to 10, resulting in a net +1 charge.
Memory Tip:
"Cats have PAWS"
Cation is a "PAW"itive ion.
Helps remember that cations are positive ions.
✅ Answer Table (Correct Answers Only)
Question | Correct Answer | Choice Text |
|---|---|---|
1 | B | An atom or group of atoms with a net electrical charge |
2 | C | By losing electrons |
3 | C | A positively charged ion |
4 | B | +1 |
❓ TEAS-Style Multiple Choice Questions (Anions and Ions)
What is an ion?
A. An atom with no charge
B. An atom or group of atoms with a net electrical charge
C. An atom with equal numbers of protons and electrons
D. An atom that always has a positive charge
How does an atom become an anion?
A. By gaining electrons
B. By losing protons
C. By losing electrons
D. By gaining neutrons
Which of the following describes an anion?
A. A positively charged ion
B. A neutral atom
C. A negatively charged ion
D. An atom with more protons than electrons
CATION VS ANION
Term | Definition |
|---|---|
Ion | An atom or group of atoms with a net electrical charge. An atom becomes an ion if it gains or loses electrons. |
ANION
Example: Oxygen (O) | Before Gaining Electrons | After Gaining Electrons |
|---|---|---|
Protons (+) | 8 | 8 |
Electrons (-) | 8 (neutral, no charge) | 10 (gained 2 electrons) |
Charge | Neutral (no charge) | Negative ion, charge = 2- (O²⁻) |
Anion is a negatively charged ion formed when an atom gains electrons.
Memory Tip:
"An anion is a negative ‘onION’."
Helps remember that anions have negative charges.
✅ Answer Table (Correct Answers Only)
Question | Correct Answer | Choice Text |
|---|---|---|
1 | B | An atom or group of atoms with a net electrical charge |
2 | A | By gaining electrons |
3 | C | A negatively charged ion |

🧪 What is a Nucleus in Chemistry?
Property |
|---|
Location ? |
Charge ? |
Mass ? |
Size ? |
Held together by ? |
In chemistry, the nucleus is the central core of an atom. It contains:
Protons (positively charged particles)
Neutrons (neutral particles with no charge)
🔑 Key Facts About the Nucleus:
Property | Details |
|---|---|
Location | Center of the atom |
Charge | Positive (because of the protons) |
Mass | Contains nearly all of the atom's mass |
Size | Extremely small compared to the entire atom |
Held together by | Strong nuclear force (overcomes repulsion between positive protons) |
🧠 Quick Analogy:
If an atom were the size of a football stadium, the nucleus would be like a tiny marble at the center—but it holds almost all the atom’s mass!
Revisit Shells, Subshells and Orbits Part 1
❓ Flashcard Questions
What subshell is available in the K shell (n=1)?
Which shell has the subshells s and p?
At which energy level does the d subshell first appear?
What is the shell name for n=4?
Which subshells are available in the N shell?
Which subshells are common to both the M and N shells?
What shell includes only the s subshell?
What is the shell name that contains s, p, and d subshells but not f?
Which energy level introduces the f subshell?
How do shells relate to energy levels in an atom?
Which electrons are involved in chemical bonding according to the diagram?
What is a subshell?
n (Energy Level) | Shell Name | Available Subshells |
|---|---|---|
n = 1 | K shell | s |
n = 2 | L shell | s, p |
n = 3 | M shell | s, p, d |
n = 4 | N shell | s, p, d, f |
Note: Only Electrons in the outermost shell participate in chemical bonds.
✅ Flashcard Answers
The s subshell.
The L shell (n=2).
At n=3, the M shell.
N shell.
s, p, d, and f.
s, p, and d.
K shell (n=1).
M shell (n=3).
Energy level n=4.
Shells are energy levels where electrons orbit the nucleus.
Only electrons in the outermost shell participate in chemical bonds.
A subshell is a subdivision of electron shells separated by electron orbitals.
Revisit Shells, Subshells and Orbits Part 2
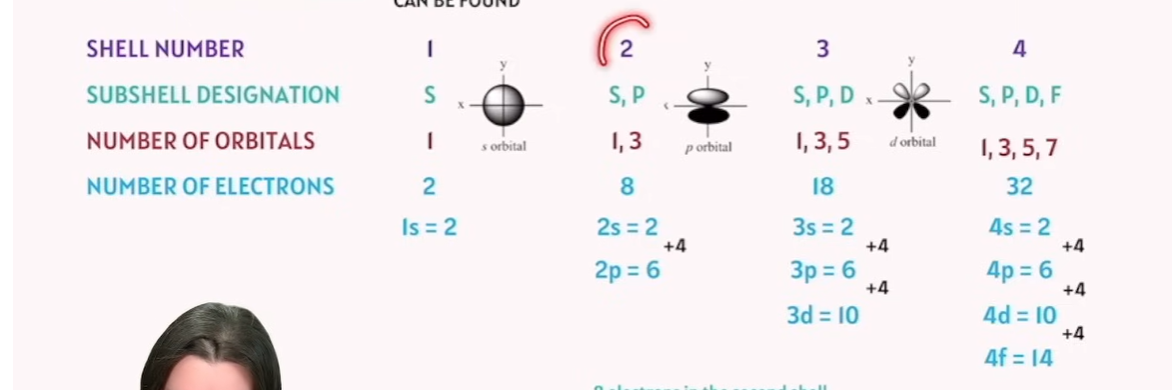
What is the subshell shape of each Shell Number
❓ Flashcard Questions
How many orbitals are in the s subshell?
What is the maximum number of electrons in shell number 1?
Which subshells are present in shell number 2?
What is the total number of orbitals in shell 3?
How many electrons can shell 3 hold in total?
What are the subshells and orbital counts in shell number 4?
Which subshell can hold up to 14 electrons?
How many electrons can fit into the p subshell of any shell?
What is the electron configuration of magnesium (atomic number 12)?
In which shell is the 3d orbital found?
How many electrons can fit in the 4f subshell?
What is the total electron capacity of shell number 4?
📊 Table: Orbital Structure by Shell Number
Shell Number | Subshell Designation | Number of Orbitals | Number of Electrons | Subshell Designation |
|---|---|---|---|---|
1 | s | 1 | 2 | |
2 | s, p | 1, 3 | 8 (2s = 2, 2p = 6) | |
3 | s, p, d | 1, 3, 5 | 18 (3s = 2, 3p = 6, 3d = 10) | |
4 | s, p, d, f | 1, 3, 5, 7 | 32 (4s = 2, 4p = 6, 4d = 10, 4f = 14) |
ATI Quiz point:
They love asking the
characteristic of the d shell/orbital: Circular Sphere
characteristic of the p shell/orbital: Dumbbell
characteristic of the d shell/orbital: Four Leaves Clover
characteristic of the f shell/orbital: Come in different Shapes
⚛ Bonus Detail:
Magnesium (Atomic Number 12) Electron Configuration:
1s² 2s² 2p⁶ 3s²
✅ Flashcard Answers
1 orbital.
2 electrons.
s and p subshells.
9 orbitals (1 from s, 3 from p, 5 from d).
18 electrons.
Subshells: s, p, d, f. Orbitals: 1, 3, 5, 7.
The f subshell.
6 electrons.
1s² 2s² 2p⁶ 3s².
In shell number 3.
14 electrons.
32 electrons.
How a chemical become stable?
Ionic Bonds
Ionic Bone: Complete transfer of valence electron(s) between Atoms
🧪 What's Happening in the Diagram?
Sodium (Na) | Chlorine (Cl) |
|---|---|
Atomic # = 11 | Atomic # = 17 |
Electron config: 2, 8, 1 → 1 valence e⁻ | Electron config: 2, 8, 7 → 7 valence e⁻ |
Not stable | Not stable |
Loses 1 electron to become Na⁺ (stable with 8 in second shell) | Gains 1 electron to become Cl⁻ (stable with 8 in third shell) |
⚛ Type of Bond: Ionic Bond
Definition: A complete transfer of electrons from one atom to another.
Sodium gives an electron.
Chlorine takes that electron.
Now, both have full outer shells.
The result is Na⁺ and Cl⁻, which are oppositely charged and attract.
This forms NaCl (table salt).
📏 Octet Rule Reminder:
Atoms are most stable when they have 8 electrons in their outer shell (except for the first shell, which is full at 2).
They might lose, gain or share electrons
🧠 Memory Tip from the Slide:
"I take, you give" → Describes the electron transfer in an ionic bond.
✅ Summary: How Ionic Bonding Leads to Stability
Sodium has 1 electron in its outer shell → gives it away → becomes stable.
Chlorine needs 1 electron to complete its outer shell → takes one → becomes stable.
This transfer creates charged ions (Na⁺ and Cl⁻) that are held together by electrostatic attraction.

Ionic Bonds Quiz:
🧪 TEAS-Style Practice Questions
# | Question |
|---|---|
1 | Which of the following is true of sodium (Na) in an ionic bond with chlorine (Cl)? |
2 | What is the total number of valence electrons in chlorine before bonding with sodium? |
3 | What type of bond forms between sodium and chlorine in NaCl? |
4 | Why does sodium form an Na⁺ ion during bonding? |
5 | What is the charge on a chlorine ion (Cl⁻) after it gains an electron from sodium? |
6 | What rule explains why atoms form chemical bonds to get 8 electrons in their outer shell? |
7 | In an ionic bond, electrons are: |
8 | After forming NaCl, how many electrons are in sodium’s outermost shell? |
✅ Answer Key
# | Correct Answer |
|---|---|
1 | C. It loses one electron |
2 | B. 7 |
3 | D. Ionic |
4 | B. To lose an electron and achieve a stable electron configuration |
5 | C. -1 |
6 | D. Octet rule |
7 | C. Completely transferred |
8 | B. 8 |
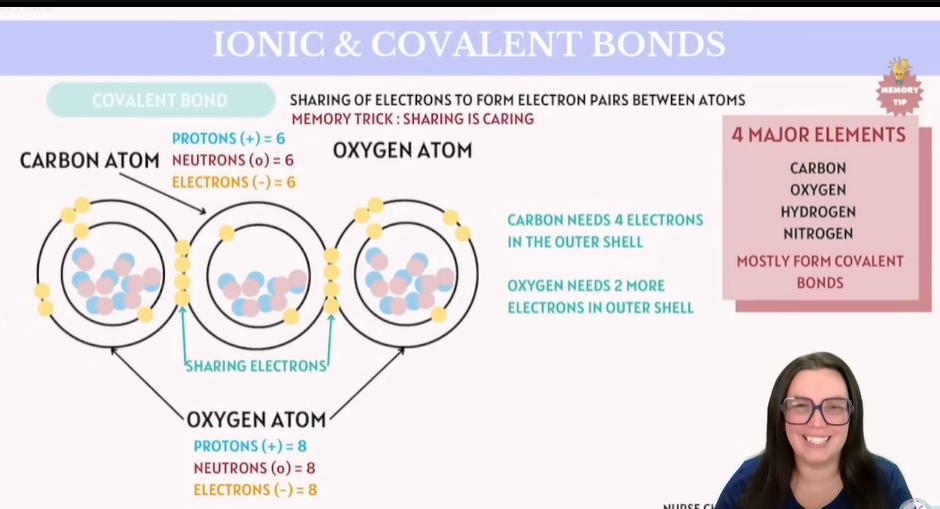
Covalent Bond
📘 TEAS-Style Questions (Covalent Bonds) Question 1:
Which of the following best describes a covalent bond?
A. Transfer of electrons from one atom to another
B. Sharing of protons between atoms
C. Sharing of electrons between atoms
D. Attraction between ions of opposite charges
Question 2:
What is the total number of electrons in a carbon atom?
A. 4
B. 6
C. 8
D. 12
Question 3:
What do oxygen atoms need in order to achieve a full outer shell?
A. 1 more proton
B. 2 more electrons
C. 4 more neutrons
D. To lose 2 electrons
Question 4:
Which of the following elements mostly forms covalent bonds?
A. Sodium
B. Magnesium
C. Chlorine
D. Hydrogen
Question 5:
How many electrons does carbon need to complete its outer shell?
A. 2
B. 4
C. 6
D. 8
Question 6:
What is the memory trick associated with covalent bonding?
A. Give to grow
B. Sharing is caring
C. Gain to maintain
D. Attract to react
Category | Details |
|---|---|
Bond Type | Covalent Bond |
Description | Sharing of electrons to form electron pairs between atoms |
Memory Trick | Sharing is caring |
Carbon Atom | Protons (+): 6 |
Oxygen Atom | Protons (+): 8 |
Electron Needs (Carbon) | Needs 4 electrons in the outer shell |
Electron Needs (Oxygen) | Needs 2 more electrons in outer shell |
4 Major Elements Mostly form covalent bonds | Carbon, Oxygen, Hydrogen, Nitrogen |
Bond Type of Major Elements | Mostly form covalent bonds |
✅ Answer Key
Question | Correct Answer | Explanation |
|---|---|---|
1 | C. Sharing of electrons between atoms | This is the defining trait of covalent bonding. |
2 | B. 6 | A carbon atom has 6 protons, 6 neutrons, and 6 electrons. |
3 | B. 2 more electrons | Oxygen needs 2 electrons to complete its outer shell. |
4 | D. Hydrogen | Hydrogen, along with carbon, oxygen, and nitrogen, primarily forms covalent bonds. |
5 | B. 4 | Carbon needs 4 electrons to fill its valence shell. |
6 | B. Sharing is caring | This phrase helps remember the concept of electron sharing in covalent bonds. |
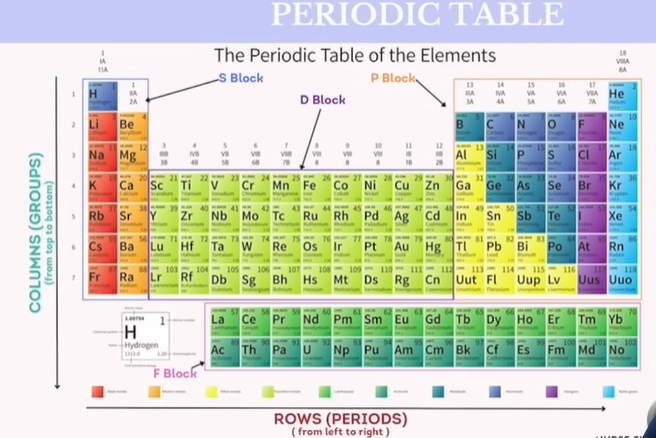
Periodic Table
For the test, we need to know what is Period & Group
🧪 What is a Period in the Periodic Table?
A period is a horizontal row in the periodic table.
There are 7 periods in total, numbered from 1 to 7.
As you move from left to right across a period:
Theatomic number increases.Elements becomeless metallicand morenon-metallic.Electrons are added to the same outer shell.
The period number of an element tells you how many electron shells (energy levels) the atom has.
✅ Example:
Carbon (C), Nitrogen (N), and Oxygen (O) are all in Period 2 – they each have electrons filling their second energy level (shell).
🧲 What is a Group in the Periodic Table?
A group is a vertical column in the periodic table.
There are 18 groups, numbered from 1 to 18.
Elements in the same group:
Have the same number of electrons in their outer shell (valence electrons).
Tend to have similar chemical properties.
✅ Example:
Group 1 = Alkali metals (e.g., Lithium, Sodium) – all have 1 valence electron and are very reactive.
Group 17 = Halogens (e.g., Fluorine, Chlorine) – all have 7 valence electrons and are also very reactive.
🔁 Quick Comparison
Feature | Period | Group |
|---|---|---|
Direction | Horizontal row | Vertical column |
Total Number | 7 | 18 |
Key Pattern | Increasing energy levels | Similar valence electron patterns |
Example | C, N, O in Period 2 | F, Cl, Br in Group 17 (Halogens) |