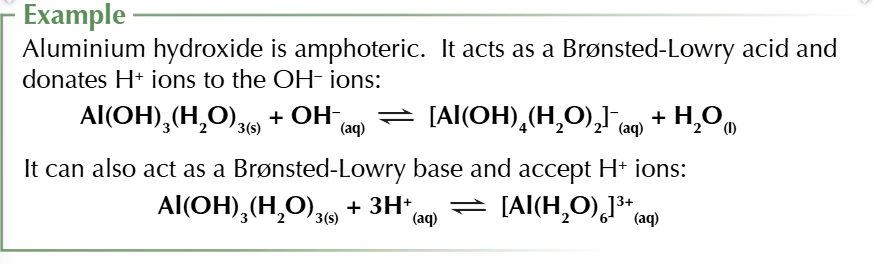2. (H2O )hydrolysis of metal aqua ions
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
1
New cards
adding OH- ions to solutions of metal aqua 3+ ions
produces insoluble precipitates of metal hydroxides
2
New cards
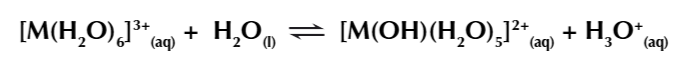
in water metal aqua 3+ ions form the equilibrium
if you add OH- ions to the equilibrium, H3O+ ions are removed shifting equ right
3
New cards

another equilibrium is now set up
OH- remove H3O+ ions from the solution pulling the equ right
4
New cards
this occurs one last time
leaving you with an insoluble uncharged metal hydroxide
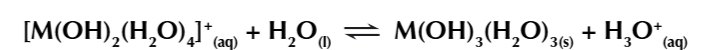
5
New cards
overall equation
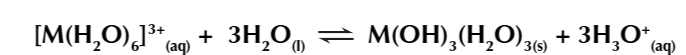
6
New cards
a similar thing happens w MA 2+ ions, only in 2 steps
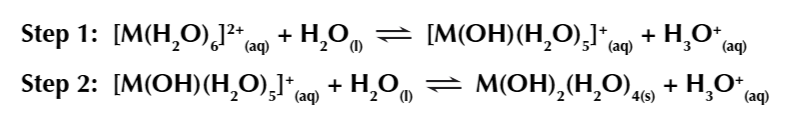
7
New cards
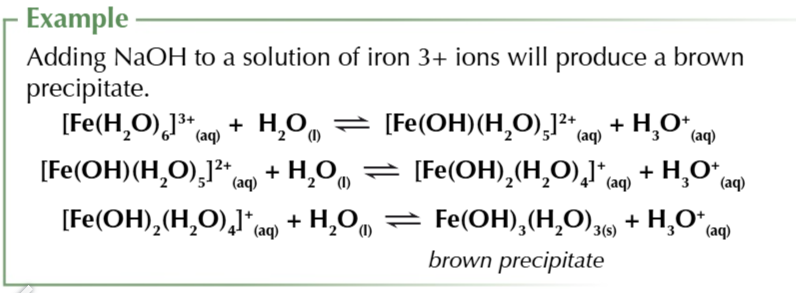
8
New cards
all the metal hydroxide precipitates will dissolve in acid
they act as bronsted lowery bases and accept H+
9
New cards
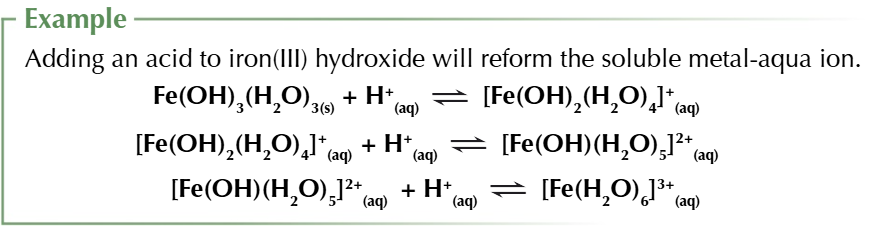
10
New cards
some metal hydroxides are amphoteric
ALUMINIUMS TRIFFLIN AHH