Orgo Lab 2 Quiz 6: Reversible Reactions
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
This week's Inquiry: Part 1
- How can we obtain the best possible yield from reversible reactions?
→ Reversible: Keq ~ 1 (both reactants and products are abundant at equilibrium)
Examples of common esters (5)
- Ethyl pentanoate: used in apple-scented products
- Isoamyl acetate: used in banana-scented products
- Bornyl acetate: component of synthetic pine-needle scent
- Ethyl acetate: produced by spoiled wine (smells bad)
- Jojoba oil: used in anti-acne skincare products
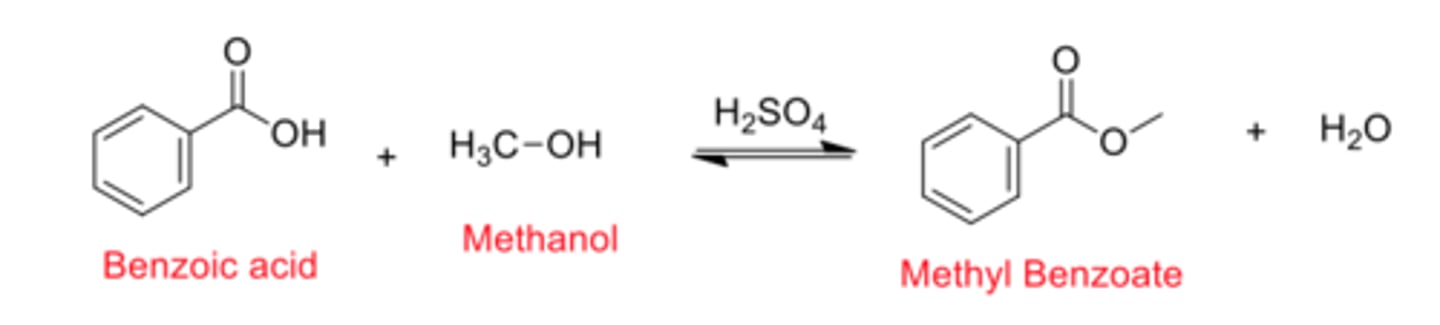
Fischer Esterification: Purpose and Mechanism
- Common method used to synthesize esters
→ Uses an acid catalyst to convert a carboxylic acid + alcohol to an ester + H₂O
Mechanism:
1. Acid catalyst protonates the carbonyl group of a carboxylic acid, making it more electrophilic
2. An alcohol (ROH) adds to the carbonyl carbon to produce a tetrahedral intermediate, which does a proton transfer, then expels H₂O
3. Net result: carbocylic acid + alcohol → ester + H₂O
What 2 reactions will we be performing? What is the goal?
PART 1:
Benzoic acid + methanol → (H₂SO₄ catalyst) → methyl benzoate + H₂O
PART 2:
Methyl benzoate + (1) 3 NaOH (2) H₃O⁺→ benzoic acid + methanol
GOAL:
- Analyze each product to see whether we achieved a high yield in both directions
What is the problem with Fischer esterification for synthesis? What is our solution?
- In Fischer esterification, all steps are REVERSIBLE, so the reaction will not naturally run to completion
- For our reaction (benzoic acid and methanol), Keq = 4.0 → this corresponds to an expected (NOT "theoretical") yield of ~67%
- Our solution: increase the concentration of a reactant (by using methanol as the solvent) to drive the reaction forward!
→ This will make the [ ] of methanol ~24.7 mol/L and will increase expected yield to ~99.7%
Why can't we use distillation to drive the esterification (methanol + benzoic acid) forward?
- The most-volatile component of our reaction mixture is methanol (BP ~ 65°C), so methanol will be preferentially removed by a fractional still
- Since methanol is a reactant, distillation would drive the reaction in reverse and DECREASE the yield of products
Our Experimental Procedure: Esterification of Benzoic Acid (Part 1)
1. Convert 5g benzoic acid to methyl benzoate using Fischer esterification
→ Combine 5.0 g benzoic acid, 25 mL methanol, and 1.5 mL H₂SO₄
2. Purify the ester product by liquid-liquid extraction with 25 mL water and 25 mL diethyl ether, then wash with 10% sodium bicarbonate and dry with anhydrous magnesium sulfate
→ Rotary evaporate to remove ether
3. Analyze the neat liquid product by FT-IR spectroscopy (to determine whether we successfully produced an ester) and ¹H NMR spectroscopy (to confirm that our product was methyl benzoate
→ Solvent: deuterated chloroform
4. Store the product in a sealed vessel for use in part 2
Chemical Waste Plan: Part 1
- All organic materials produced are NON-HALOGENATED ORGANICS
- Aqueous washings (from liquid-liquid extraction?) can be acidic or basic, so test pH using alkacid paper, then place in AQUEOUS OR BASIC ACIDIC container as appropriate
- Transfer pipettes = BROKEN GLASS
- Filter paper should be dried in the hood, then placed in SOLID WASTE
This week's Inquiry: Part 2
- How can we make benzoic acid from methyl benzoate?
→ We are converting last lab's product back into the original reactants via saponification
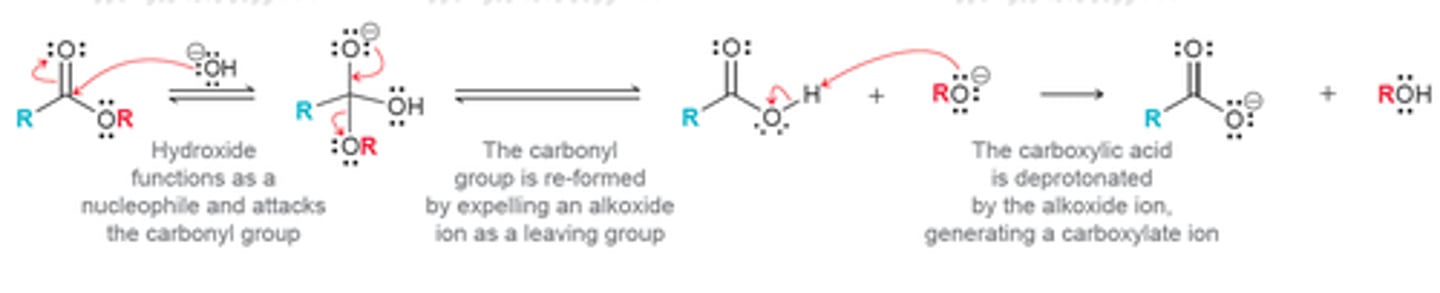
What are 2 pathways for the hydrolysis of esters?
1. Acid-catalyzed hydrolysis
- Yields a CARBOCYLIC ACID and an ALCOHOL
- Basically the reverse of the Fischer esterification process (and, thus, establishes an *equilibrium*)
2. Hydrolysis under basic conditions ("saponification")
- Yields a CARBOXYLATE (deprotonated carboxylic acid) and an ALCOHOL
- Once the OH⁻ adds and is deprotonated, the reaction is *NOT reversible* (because nucleophiles will not attack the anionic carboxylate)
- This means that the reaction should proceed to completion!
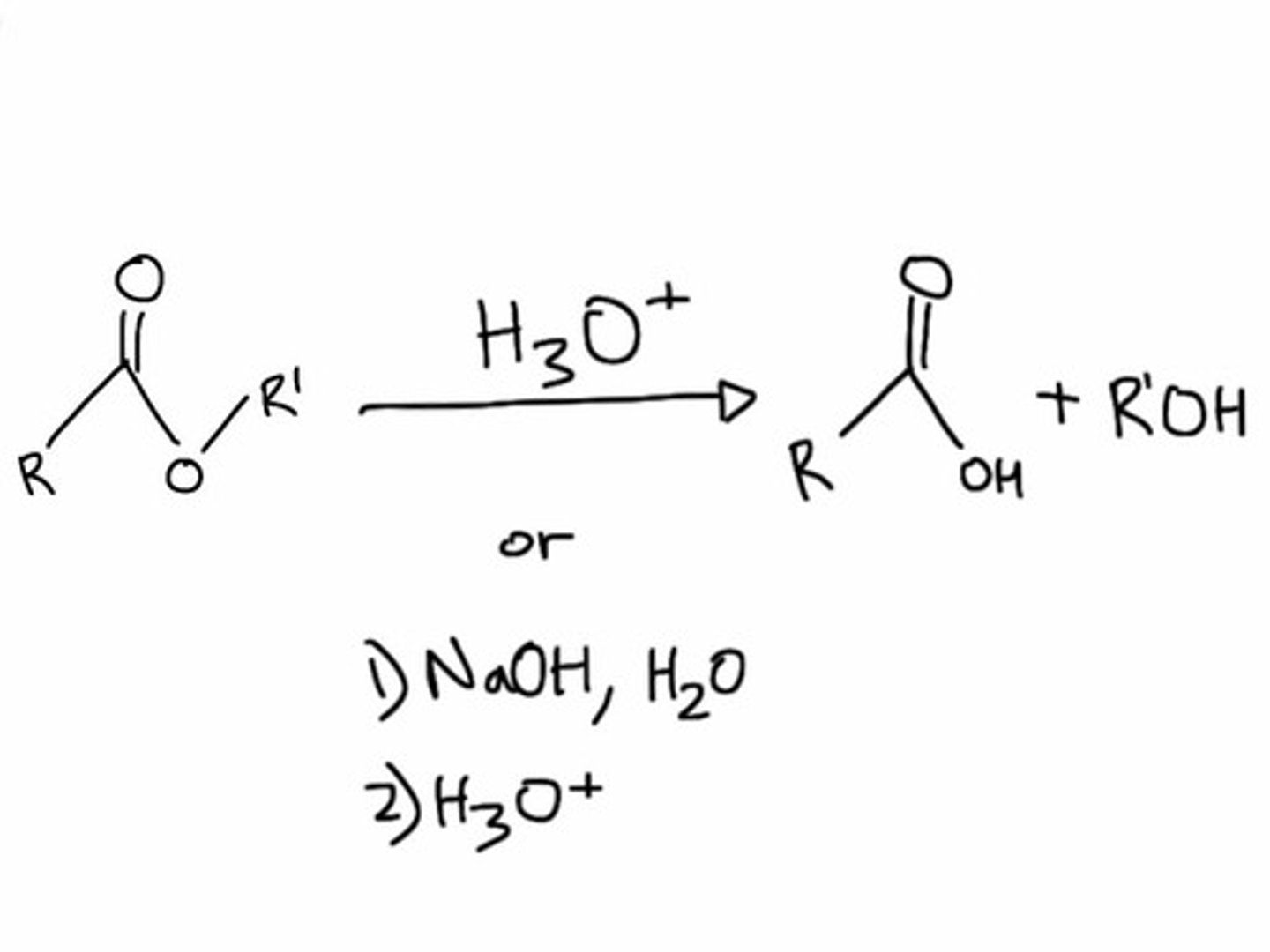
Saponification: definition and process
- The clevage of an ester into an alcohol + carboxylate anion using H₂O and BASE
- Note: NaOH (the base) is CONSUMED in this reaction (adds to the carbonyl carbon) → it is not acting as a catalyst
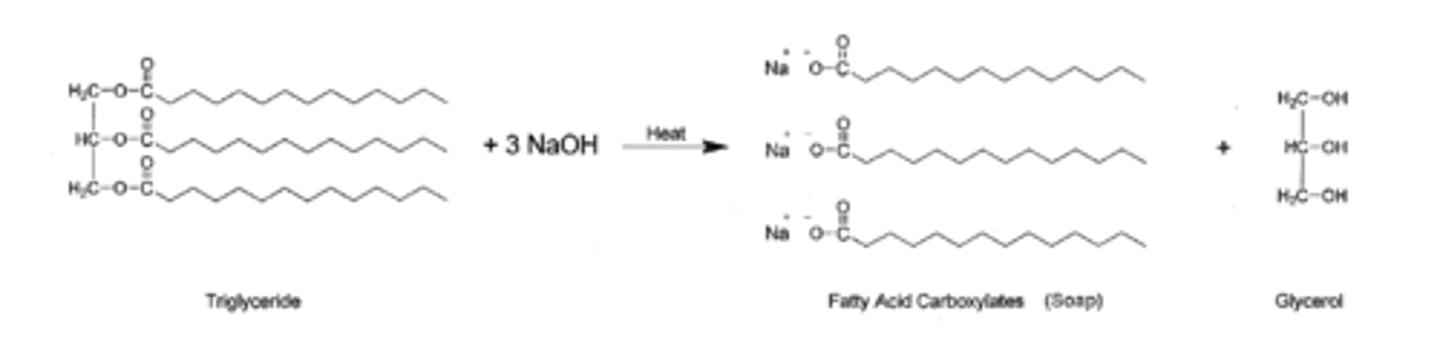
Modern uses of saponification
- The fatty acids produced by hydrolysis of triglycerides (esters!) are common ingredients in detergents and soaps
Fat (solid lipid) or oil (liquid lipid) + 3 equivalents NaOH → glycerine/glycerol + 3 fatty acid salts (carboxylic acid compounds)
How was saponification/soap discovered (according to the Fight Club dude?)
- Wood ashes + water = lye (basic)
- Lye + fat (from burnt bodies) = soap
- Also: glycerine (byproduct of triglyceride saponification) + HNO₃ = nitroglycerine (explosives)
*In case prof Davis asks, this guy's name is Tyler Durden
Safety notes: silica glasswear
- Silica (in glass) is slightly soluble in bases—when refluxing a very basic solution, you may prioduce NaSiO₃ (a component of cement), which can "cement" your glasswear together
- Solution: use extra vacuum grease
What should we look for in the IR when analyzing our products?
- Part 1: if we converted benzoic acid into methyl benzoate, the broad carboxylic acid O-H absorption should disappear
- Part 2: if we re-produced benzoic acid, the O-H should be present
Our experimental procedure: part 2
1. Convert 5.7 g of methylbenzoate into benzoic acid using a saponification reaction
→ Combine 5.7 g methylbenzoate and 40 mL of 3M NaOH, and reflux for 45 minutes
2. Precipitate the benzoic acid product by acidifying the reaction mixture with HCl
→ Produces benzoic acid from benzoate ions)
3. Recrystalize the precipitate from deionized water
4. Analyze the product by FT-IR and ¹H NMR spectroscopy
Chemical Waste Plan: Part 2
- All organic materials are NON-HALOGENATED ORGANICS
- Aqueous solutions = AQUEOUS ACIDIC
- Filter paper (dried in fume hood) = SOLID WASTE
- Transfer pipettes = BROKEN GLASS WASTE