Che 001 Midterm - UC Davis
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
NH₄⁺
Ammonium
OH⁻
Hydroxide
NO₃⁻
Nitrate
SO₄²⁻
Sulfate
CO₃⁻²
Carbonate
PO₄³⁻
Phosphate
C₂H₃O₂⁻
Acetate
HCO₃⁻
Bicarbonate
ClO₃⁻
Chlorate
CN⁻
Cyanide
NO₂⁻
Nitrite
ClO₄⁻
Perchlorate
MnO₄⁻
Permanganate
CrO₄²⁻
Chromate
O₂²⁻
Peroxide
SO₃²⁻
Sulfite
What are the 2 types of pure substances?
elements and compounds
In clockwise order, what are the 5 things listed for each element on the periodic table?
Atomic mass, symbol, chemical group block, name, and atomic number
What are the most abundant elements on earth?
Main group elements
What type of element is found in columns 1, 2, and 13-18?
Main group elements
What type of element is found in columns 3-12?
Transition metals
What is a period (on the periodic table)
Elements in the same row
What is a group on the periodic table?
Elements in the same column
Do groups or periods share similar properties?
Groups
True/False: most of the elements on the periodic table are nonmetals
False— most are metals
What are the 3 characteristics of metals
1: solidifies at room temp
2: shiny in appearance
3: good conductors of heat and electricity
What are the 4 characteristics of nonmetals?
1. Can be solid, liquid, or gas at room temp
2. Normally dull in appearance
3. Poor conductors of heat and electricity
4. Cannot be deformed without breaking or cracking
What type of mixture has uniform composition?
Homogenous
What kind of mixture is not uniformly distributed in space? Composition varies from spot to spot
Heterogenous
Species that contain single atoms as fundamental units
Monatomic species
Pure substances and mixtures are examples of what?
Matter classified by composition
Species that contain molecules with multiple atoms (same or different elements) as fundamental units
Molecular species
Liquid....have a fixed shape
Liquid...adapt to the shape of a container
Liquid...a fixed volume
Does not, can, has
Solids have a...shape
Solids have a...volume
Fixed, fixed
Gas has a...shape
Gas has a...volume
Indefinite, indefinite
What type of matter has closely packed particles with little freedom to move>
Solid
What type of matter has randomly arranged particles with freedom to move?
Liquid
What does (s), (l), (g) and (aq) mean?
solid, liquid, gas, aqueous (dissolves in water)
A change with no alteration to the chemical composition is a
Physical change
A change which involves the formation of new substances is a
Chemical change
What are intensive properties?
Properties that exist regardless of scale: density, color, temperature etc
How do you convert Celsius to Kelvin?
C+273.15=K
How do you convert Fahrenheit to Celsius?
(F - 32) / 1.8 = C
How do you convert Fahrenheit to Kelvin?
First convert F to C. Then add 273.15
How do you convert Celsius to Fahrenheit?
(C x 1.8) + 32 = F
How do you convert Kelvin to Fahrenheit?
First: (K-273.15) = C
Second: Cx1.8+32 = F
matter
Anything that has mass and takes up space
Atoms
smallest builiding blocks of matter
Ions
variation of atoms with added or subtracted electrons
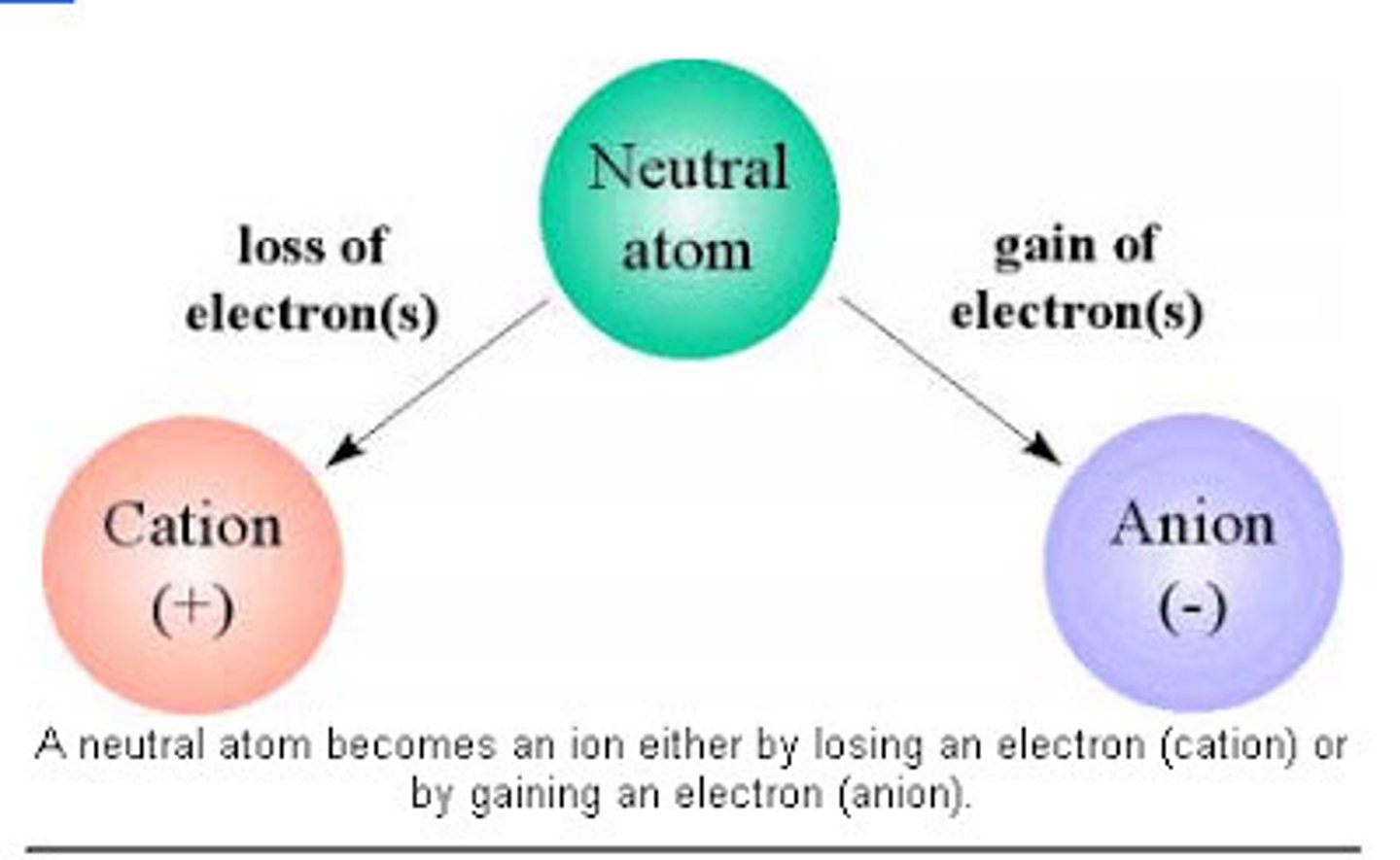
Anion
negatively charged ion (gained electrons)
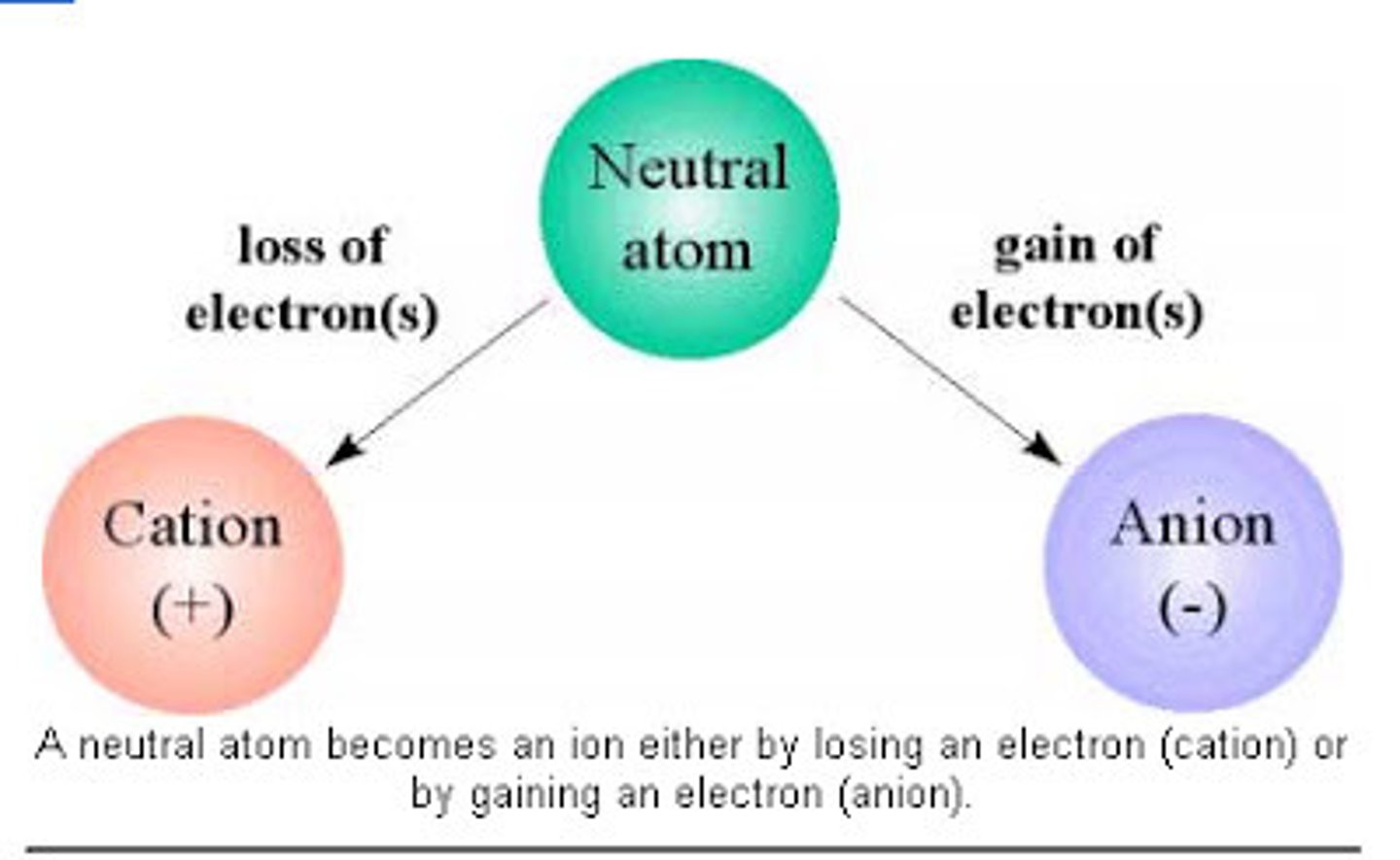
Cation
A positively charged ion (loose an electron)
Molecules
Groups of two or more atoms held together by chemical bonds
elements
pure substance that consists entirely of one type of atom
Compounds
2 or more elements chemically combined
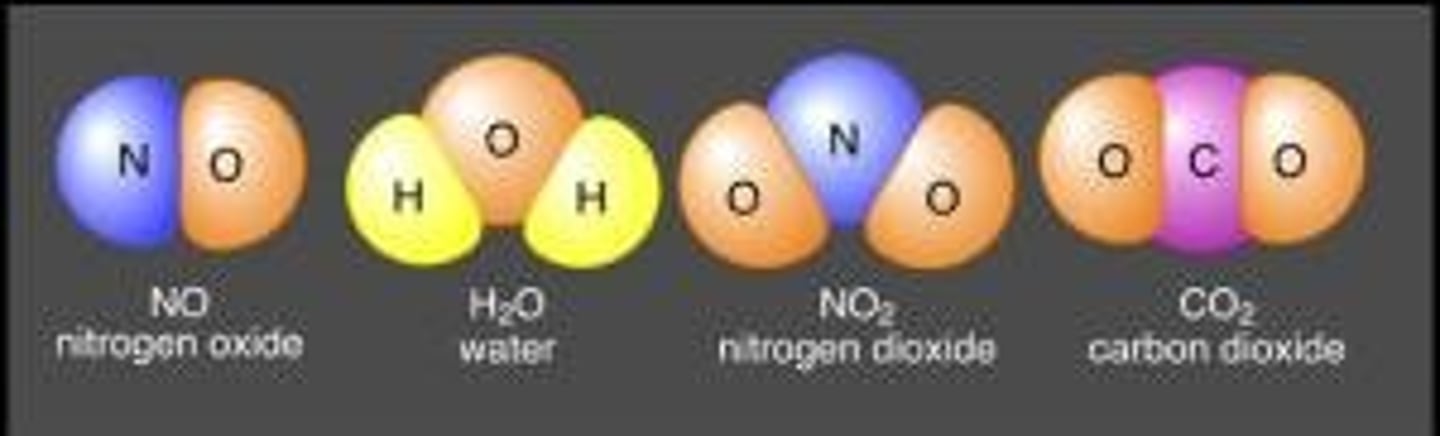
molecular compounds
composed of two or more non metals
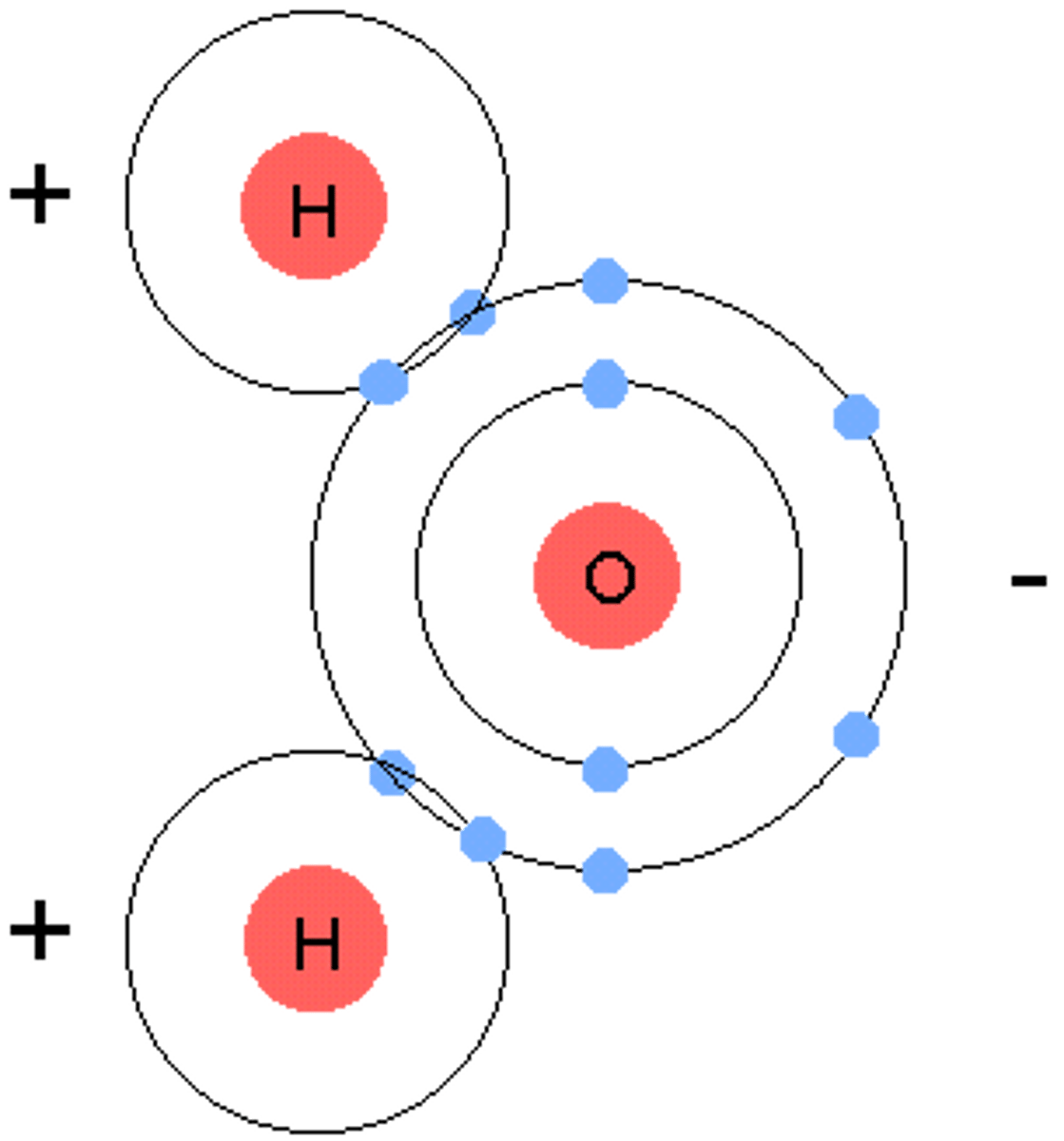
ionic compounds
when a non metal and metal combine
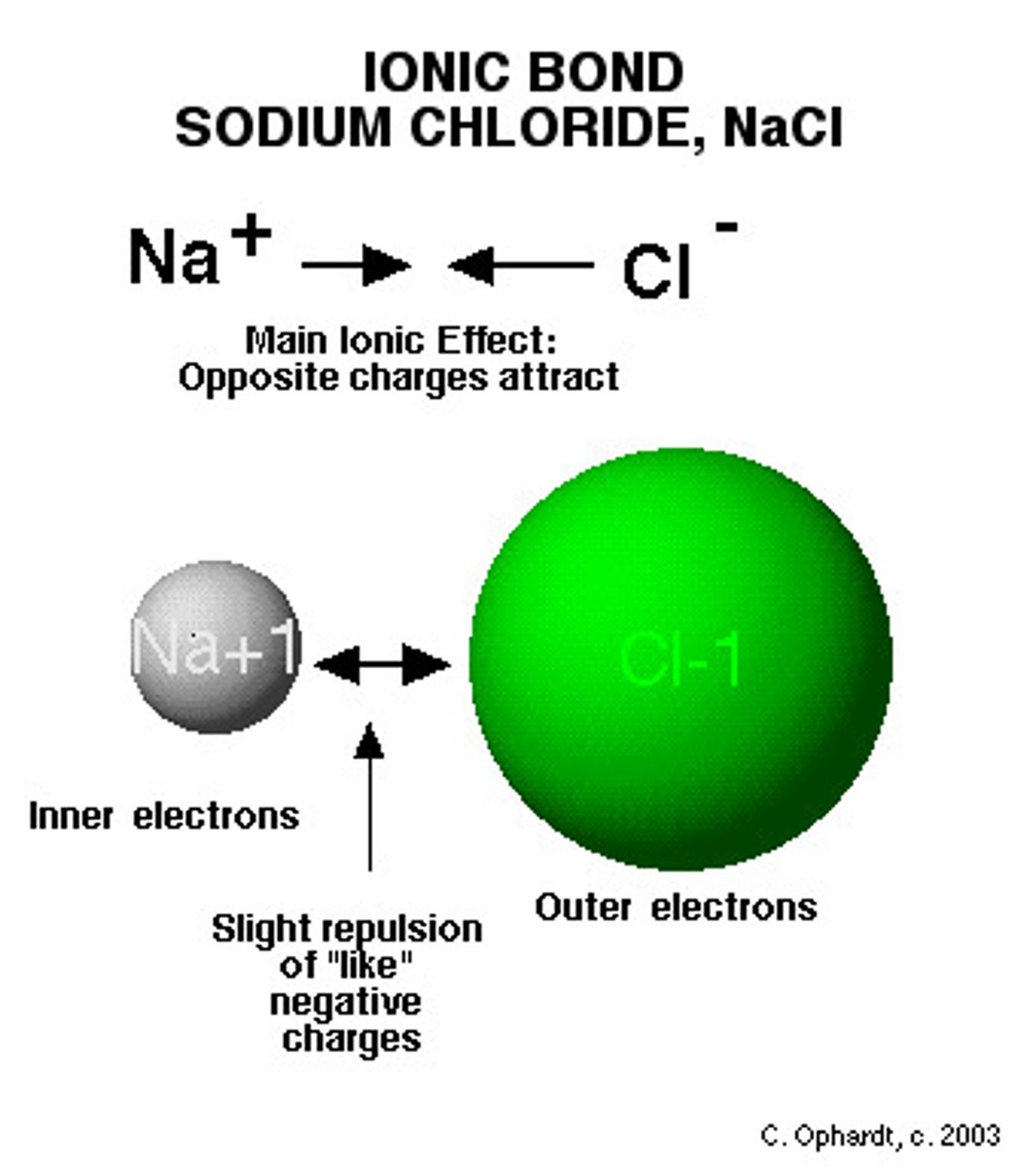
Mixtures
combonation of two+ pure substances
substances
A class of matter made up by elements and compounds.
Homogenous mixtures
uniform in composition
Heterogenous mixtures are...
not uniform in composition
Per
(prefix) one more oxygen than "ate"
Hypo
(prefix) 1 less oxygen than "ite"
ide
monoatomic
ite
1 less oxygen (2)
ate
1 more oxygen (3)
alkali metal charge and row
first row plus 1
alkaline earth metals row and charge
second row charge 2
group thirteen charge
3
chalcogens charge
-2
halogens charge
-1
noble gas charge
0
avo number
6.02241 × 10²³ mol ^ -1