Animal Reproduction Exam 3 (Rutgers)
1/200
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
201 Terms
Major sequence of events following deposition of sperm in female tract
1. immediate transport
2. cervix
3. uterus
4. oviduct
5. fertilization
immediate transport of sperm
retrograde loss, phagocytosis, entrance into cervix/uterus
15 minutes
average time between coitus and arrival of sperm in oviducts
1.5-6 days
average fertile life of sperm
8-24 hours
average fertile life of egg
sperm loss is dependent on
1. physical nature of the ejaculate
2. phagocytosis
3. contractility of reproductive tract
4. cervical secretions and the ability of sperm to navigate through two types of cervical mucus
Fallopian tube/salpinx
paired muscular tubes containing: infundibulum (fimbriae, cilia-oocyte cumulous complex 'pick-up'), ampulla, isthmus
ampulla
site of fertilization in the oviduct
site of sperm deposition: cervix
pigs, horses, camelids
site of sperm deposition: vagina
cow, sheep, rabbit, rodents, primates, dogs & cats
pig/horse ejaculate
final fraction highly viscous "rice pudding"
rodent ejaculate
coagulating protein in semen forms a vaginal plug = mating marker
phagocytosis
neutrophils (leukocytes or WBCs), during estrus, after insemination, first line responders to infections and attacks "foreign" proteins==> causes sperm loss & prevents tract infection
contractility of the reproductive tract
Estradiol (high at insemination), oxytocin (released at coitus), prostaglandins (f2a and E1) ==> causes increased motility of oviduct and uterus
Administering Phenylephrine or Ergonovine
reduces retrograde sperm loss (increased % fertilization)
Sulfomucin
high viscosity, apical barrier to sperm; eliminates non-motile sperm
Sialomucin
low viscosity, in basal areas of cervical crypts sperm enter --> reservoir = silo; sperm easily swims through
artificial insemination in uterine body vs. uterine horn
no difference in cumulative % recovered sperm
pig/camelids ejaculation
seminal fluid can induce ovulation
humans & bull ejaculation
seminal fluid has pH ~6.7-7.4; neutralizes vaginal acidity (pH~4)
AI in cervix vs. uterine horn
much more sperm recovered in cervix
intracervical insemination
used in pigs, within the cervix
transcervical insemination
used in cows, bypass the cervix
What kills sperm?
low pH, mucins, neutrophils, retrograde loss, sperm-sperm competition
What helps sperm?
seminal fluid pH, ovulation trigger, smooth muscle stimulants, sheer numbers
Chemotaxis (marine species)
resact- sea urchin peptide emitted by the egg, increases sperm motility
chemotaxis (mammals)
sperm attracted to follicular fluid, eggs & cumulous complexes, dependent on capacitation
thermotaxis
sperm can orient in the thermal gradient within the oviduct, sperm becomes hyperactive at increased temperatures (40C)
Sperm maturation
series of changes that render sperm competent to fertilize the egg, sperm ____ in the epididymis but require an extra step to fertilize the egg
Capacitation (steps)
1. epididymal- surface proteins and CHO + seminal plasma
2. ejaculated- seminal plasma coats the surface proteins + female tract
3. ______ - seminal plasma coating and some surface proteins removed
capacitation (requirements)
1. occurs in the female tract
2. NOT species specific
3. can be induced in vitro
4. reversible: _____ sperm + seminal plasma = sperm de_______
capacitation
sperm proteins removed from the sperm head
Why is capacitation important?
holds sperm in check, prevents penetration of epididymis, vaginal wall, as seminal plasma is removed, sperm gain ability to penetrate the oocyte
post-capacitation sequence of events leading to fertilization
1. hyperactive motility
2. binding to zona pellucida
3. acrosomal reaction
4. penetration of ZP
5. sperm-oocyte membrane fusion
6. sperm engulfed
7. decondensation of sperm nucleus
8. formation of mature male pronucleus
sperm hyperactivity in the oviduct
motility patterns change from linear movement to frenzied motion in the oviduct, facilitates sperm-oocyte contact, controlled molecular reaction
sperm-egg recognition
vital for fertility, blocks polyspermy; ZP3 is the main receptor
acrosomal reaction
1. sperm PM contains 2 'receptor-like' regions
2. both bind to ZP3
3. ZBR: secures sperm
4. ARPR: initiates release of acrosomal enzymes
when does the acrosomal reaction occur?
1. sperm binds to ZP
2. acrosome rxn begins enzymatic drill thru the ZP leakage of acrosomal enzymes from the sperm head
3. penetration of the ZP: loss of acrosome outer membrane
4. sperm-oocyte fusion: sperm head penetrates oocyte plasma membrane
5. cortical reaction: exocytosis of cortical granules to harden the ZP, block to polyspermy
Fusion events
1. complete penetration
2. fusion begins
3. nucleus breaks apart
zona block
coritcal reaction creates barrier at ZP level
polyspermy
fertilization of oocyte by more than one sperm, resulting in embryo death
viteline block
cortical run can alter oocyte membrane, resulting in a _______ to prevent additional sperm fusion
after sperm/oocyte fusion
sperm nucleus "decondenses" (disulfide x-links reduced, chromosomes pair)
syngamy
moment of fertilization, fusion of male and female pronuclei, zygote formed
electrical
fast block to polyspermy in non-mammalian
cortical granules
slow block to polyspermy in mammals
embryo
early stages of development; ____ of all species appear similar
fetus
unborn young still within the uterus species recognizable
conceptus
products of conception
1. embryo (during embryonic stage)
2. embryo + extraembryonic membranes
3. fetus + placenta
prior to embryo attachment
1. development within the ZP
2. 'hatching' of the blastocyst
3. extraembryonic membrane formation
4. maternal recognition of pregnancy
totipotent
ability to give rise to a complete individual
preimplantation development
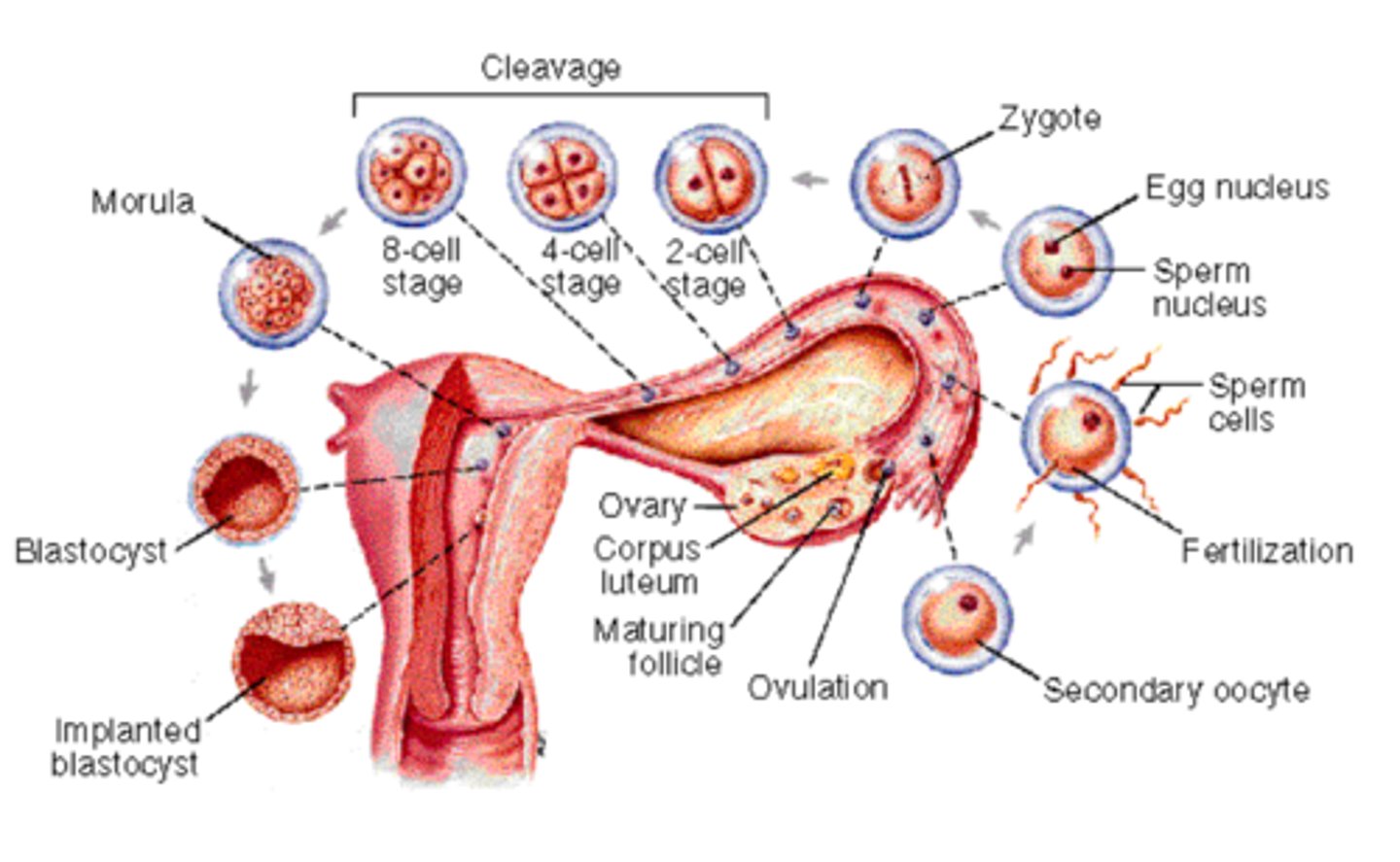
steps to blastocyst 'hatching'
1. as the ____ grows and fluid accumulates, pressure rises
2. trophoblast cells begin producing enzymes, ZP weakens
3. ____ begins to contract and relax in pulses
4. ZP ruptures- embryo is free-- floating in uterine lumen
SCNT
somatic cell nuclear transfer; source of embryonic stem cells
conceptus growth
occurs in cow, pig, sheep, spherical > tubular > filamentous *mare remains spherical
four extra embryonic membranes
chorion (1), amnion (1), yolk sac(2), allantois (4)
extraembryonic membranes originate from
trophoblast, mesoderm, embryo, endoderm
yolk sac
cavity formed by the endoderm, regresses as embryo develops, contributes blood cells, primordial germ cells
mesoderm
grows and surrounds yolk sac, pushes against the trophectoderm to form amniotic folds
chorion
fusion of mesoderm and trophectoderm, forms fetal site of placental attachment
amnion
fluid-filled sac formed by the chorion, surrounds and protects embryo
allantochorion
fusion of the two membranes from the allantois & chorion, fetal contribution to the placenta
allantois
develops from the embryonic gut and collects liquid wastes
maternal recognition of pregnancy signal
usually biochemical, may also be mechanical, species-specific ==> prevents luteolysis, maintains high P4
embryonic signal in cows and sheep
interferon-tau, secreted by embryonic trophoblast cells into the uterine lumen day 13-21 of pregnancy; decreases production of OT receptors (OT cannot stimulate PGF2a synthesis); promotes protein synthesis in gonads--> promotes implantation
embryonic signal in pigs
estradiol; causes exocrine secretion of PGF2a into the uterine lumen where it is destroyed;
also need a mechanical signal, otherwise PGF2a is secreted by one of the horns and pregnancy ends
embryonic signal in horses
use mechanical signals; conceptus migrates between horns, multiple contacts with endometrium
embryonic signal in primates/women
hCG (human chorionic gonadotropin)[inhibits luteolysis, LH-like] initially secreted 8-10 d post conception by blastocyst trophectoderm; as placenta forms, chorion cells produce hCG, basis for rapid immunoassay & pregnancy diagnosis
attachment
conceptus forms a close relationship with endometrium but does NOT embed in the uterine wall; occurs in all domestic species and monkeys
implantation
conceptus embeds in uterine wall in contact with vascular and connective tissue on all sides; occurs in higher primates (chimps & man), rodents (guinea pigs, hedgehogs, rat)
delayed implantation
blastocyst floats in uterine lumen until attachment/implantation; ensures young are born at the right time (usually spring); occurs in bears, roe deer, mink, weasels, badgers, seals, sea mammals
factors involved in delayed implantation
1. light or increasing day length stimulates implantation
2. lactation delays implantation
embryo transfer
all farm animals; embryos from a donor mother can be transferred to other females for development to term; benefits: amplify number of offspring that donor females with desired traits can produce
steps in embryo transfer
1. sync of recipients with donor
2. super ovulation of donor
3. inseminate donor with semen from genetically superior bull
4. recovery & identification of viable embryos (uterine flushing)
5. transfer of viable embryos into synchronized recipients
6. pregnancy detection/ birth of calf
window of uterine receptivity
limited period of time when the uterus is able to support blastocyst attachment and implantation
preceptive
uterus unresponsive blastocysts
receptive
ovarian P4 priming followed by estrogen
nonreceptive (refractory)
high estrogen
bruce effect
hilda ____ reported that exposure of early pregnant mice to a novel male induces implantation failure (~80% of loss)
blastocyst implantation
vulnerable to excretion of estrogens by novel males
bruce effect: steps
1. inc in E2 can result in implantation failure
2. males secrete E2 in urine in response to females
3. E2 in male urine can be absorbed byVMN in females and target the uterus
4. contributing to implantation failure
placenta
temporary relationship with uterus= significant advantage to conceptus; provides: adequate nutrition, protection from environmental danger
oviparous
egg laying
eutherian mammals
subdivision of mammals with a placenta
which orders of mammals do not have a placenta?
1. marsupials (kangaroos, koalas, etc.) 2. monotremes (platypuses)
chorionic villi
finger-like projections from surface of the chorion, interact with uterine endometrium, distribution of ____ can be used to classify type of placenta
Diffuse placenta
uniform distribution of chorionic villi
found in: sow, mare
greatest surface area
cotyledonary placenta
cotyledon= placental unit of trophoblastic origin (fetal)
placentome= point of interface
chorionic villi in clumps
found in: cattle, sheep, goats (ruminants)
zonary placenta
broad band around chorion near the middle of the conceptus, bordered by pigmented ring of small hematomas (blood clots of unknown function)
found in: cats, dogs
discoid placenta
characterized by presence of 1 or 2 disk-like structures on the chorion, discs contain chorionic villi
found in: primates, rodents
least surface area
placental classification
described by the number of layers of placenta separating maternal blood from fetal blood
prefix= maternal suffix=fetal
Epthileliochorial placenta
6 layers- 3 maternal, 3 fetal
least efficient, lack of intimate relationship offset by large surface area of diffuse placenta
found in: mare & sow
LEAST invasive
syndesmochorial placenta
5 layers: 3 fetal, 2 maternal
found in: ruminants
binucleate giant cells
unique to ruminant placenta, originate from the fetal trophoblast, migrate & invade maternal endometrial epithelium, transfer molecules from fetus to mom; secrete: placental lactogen, steroids, pregnancy specific protein B
Endotheliochorial placenta
4 layers: 3 fetal, 1 maternal
fetal chorionic epithelium in contact with maternal capillary
found in: cats & dogs
Hemochorial placenta
3 layers: fetal only
fetal chorionic epithelium in direct contact with maternal blood
found in: primates
Hemoendothelial placenta
1 layer: fetal endometrium
complete erosion of maternal layers, fetal capillaries in direct contact with maternal blood
found in: rabbit, rat, guinea pigs
MOST invasive
Placental exchange
simple diffusion (water, blood gases), active transport (pumps: sodium, potassium, calcium), facilitated diffusion (glucose [fetal energy source of mostly maternal origin], amino acids)
What WILL pass the human placental exchange?
steroids, RBCs, H2O soluble vitamins, minerals, immunoglobulins (exceptions), alcohol, drugs, bacteria, viruses (measles, HIV), metals (lead, mercury)
What will NOT pass the human placental exchange?
fat soluble vitamins, lipids, maternal proteins, large peptide hormones (TSH, ACTH, GH, insulin)