food tests and calibration curves
1/12
Earn XP
Description and Tags
no calibration curve detail - watch primrose kitten video because i can't explain it to save my life
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
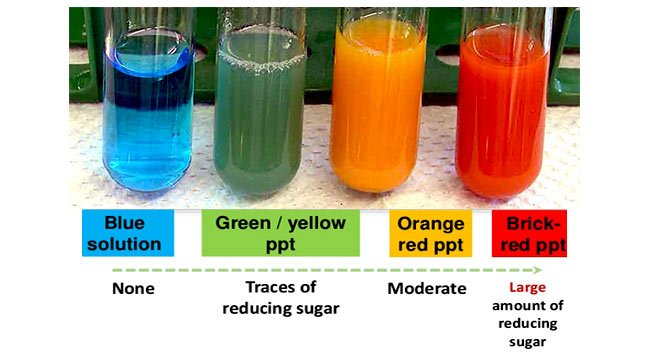
how can you test for reducing sugars?
(typical) Benedict’s test:
place 2 cm³ of liquid testing substance in a boiling tube (if not liquid, first crush w/ pestle and mortar and add distilled water, then use filter paper and funnel to create a filtrate)
add 10 drops of Benedict’s
place in a boiling water bath for 3-5 mins
if positive - blue → green/yellow/orange/brick red precipitate (depending on conc of reducing sugar)
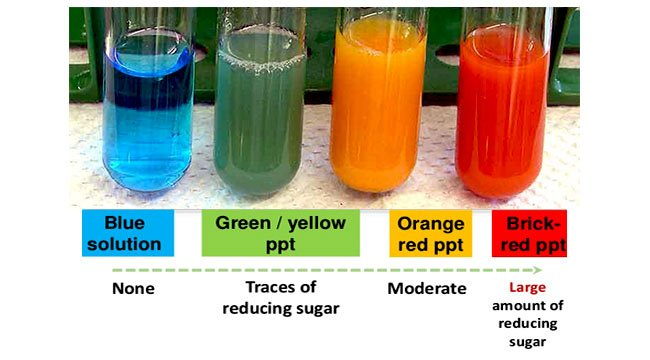
how can you test for non reducing sugars?
(altered) Benedict’s test:
boil in diute HCl (to hydrolyse the non-reducing sugar)
add sodium hydrogen carbonate (to neutralise solution)
place 2 cm³ of liquid testing substance in a boiling tube (if not liquid, first crush w/ pestle and mortar and add distilled water, then use filter paper and funnel to create a filtrate)
add 10 drops of Benedict’s
place in a boiling water bath for 3-5 mins
if positive - blue → green/yellow/orange/brick red precipitate (depending on conc of non reducing sugar)
name the reducing sugars:
(all monosaccharides)
glucose
galactose
fructose
maltose
lactose
name a non reducing sugar:
lactose
what is the difference between a reducing and a non reducing sugar?
reducing sugars act as reducing agents in chemical reactions, whereas non reduicng sugars do not
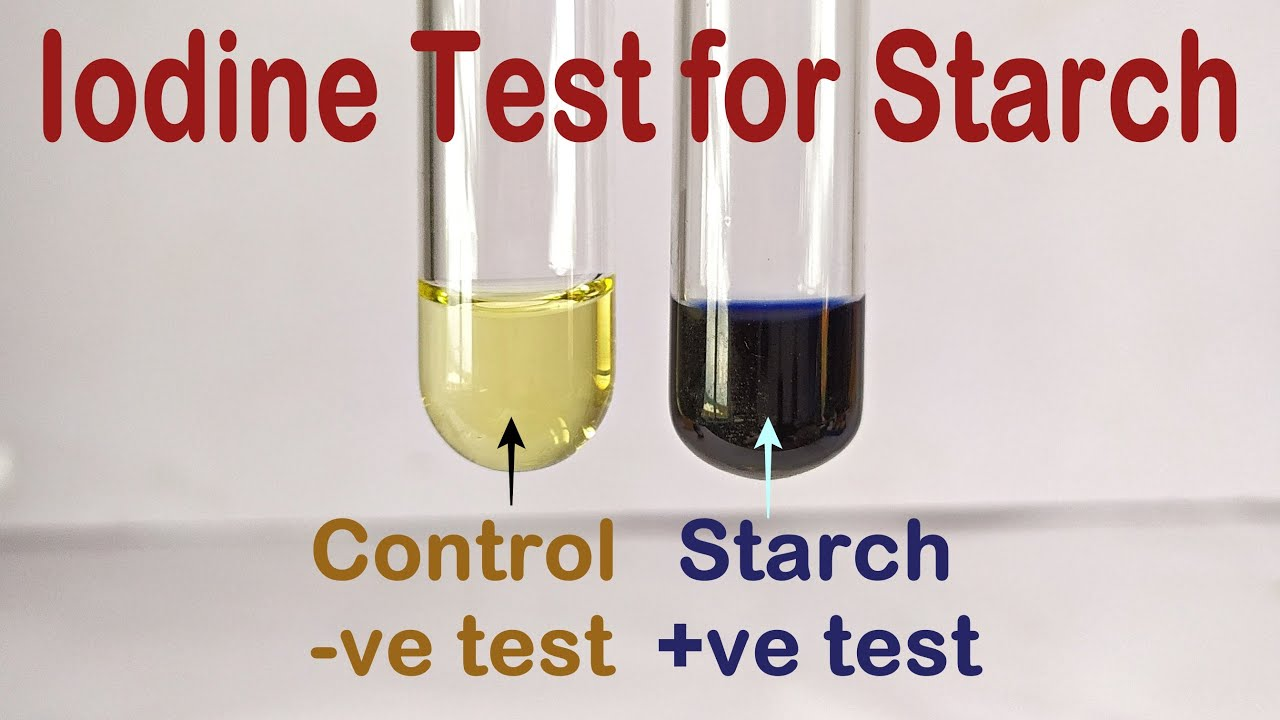
how can you test for starch?
iodine test:
place 3 cm³ of liquid testing substance in a boiling tube (if not liquid, first crush w/ pestle and mortar and add distilled water, then use filter paper and funnel to create a filtrate)
add 1 cm³ of iodine solution (containing iodine and potassium iodide)
if positive - orange → blue black
how can you test for lipids?
emulsion test:
place 3 cm³ of liquid testing substance in a boiling tube (if not liquid, first crush w/ pestle and mortar and add distilled water, then leave mixture for a while to allow particles to settle)
add 3 cm³ of ethanol
add 3cm³ of water
shake solution
if positive - clear → milky white emulsion

why do we not filter the lipid solution when testing for lipids?
lipids may stick to filter paper
how can you test for proteins?
biuret test:
place 3 cm³ of liquid testing substance in a boiling tube (if not liquid, first crush w/ pestle and mortar and add distilled water, then use filter paper and funnel to create a filtrate)
add 3 cm³ of dilute NaOH solution and mix
add 10 drops of dilute copper (II) sulfate solution and mix
(this is biuret solution - may be premixed)
if positive - blue → violet

why would you get a negative result in the biuret test for a solution w/ just amino acids (and not proteins)?
tests for peptide bonds (which would not be present)
what is a calibration curve used for?
to demonstrate the conc of a substance in an unknown sample
creatinine detecting solution reacts w/ creatinine to produce an orange colour - how could you produce a calibration curve for creatinine?
use distilled water and creatinine solutio to produce a serial dilution
add creatinine detecting solution to each soltuion
use a known/specified/constant vol of a soltuion (e.g. diluted creatinine solution)
record absorbance/transmission of solution(s)using a colourimeter
plot dilution/conc of creatinine solution against absorbance/transmission
summarise how a colourimeter works
colourimeter detects how much light is absorbed by solution (how much of the light that is travelling through the solution is being stopped by the solution)
the more concentrated the solution, the more light it will absorb
we can use this to determine to conc of a solution