850 Exam 1 (pka and bond ionization)
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
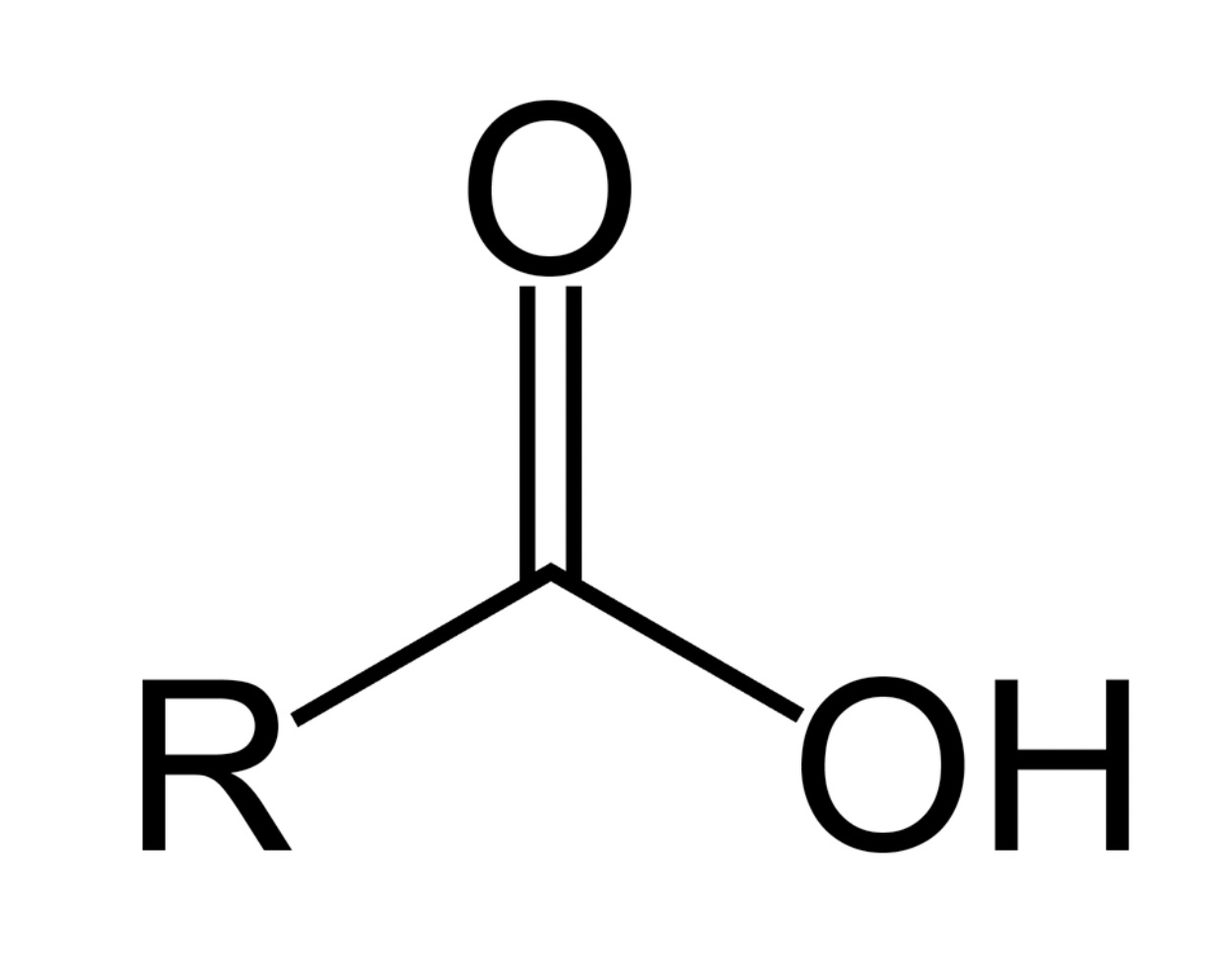
Carboxylic Acid pka?
pka is approximately 5
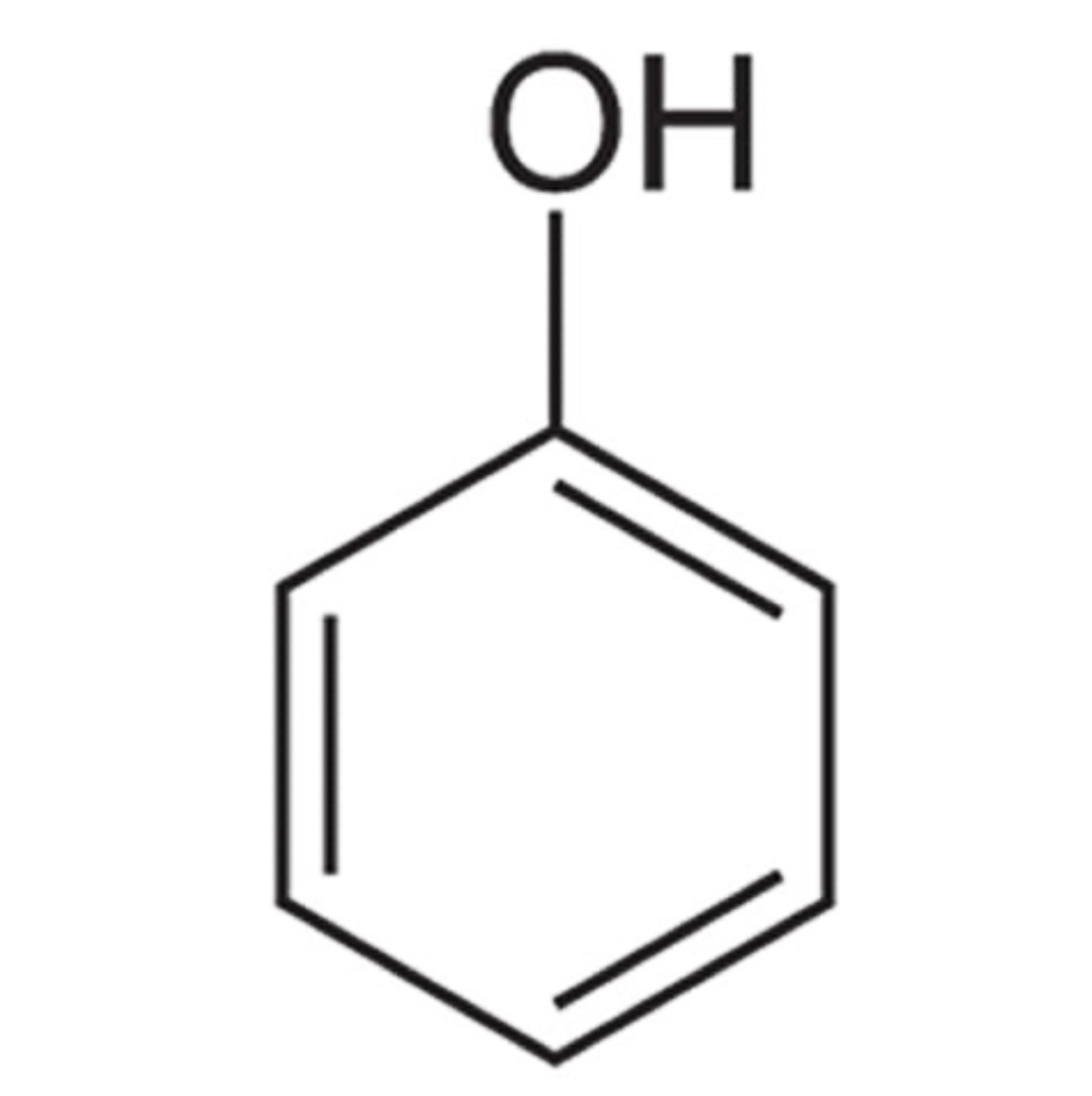
Phenol pka?
pka is approximately 10
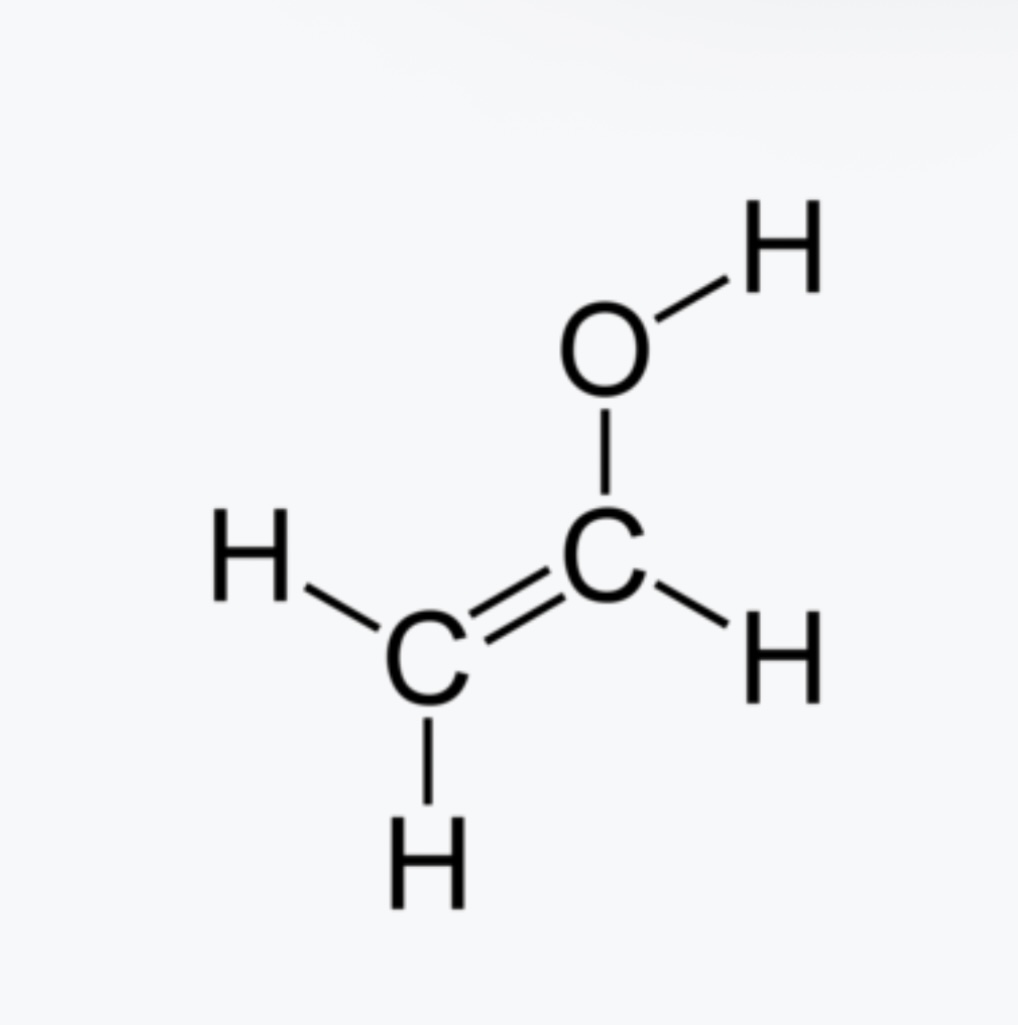
Vinyl Alcohol pka?
pka is approximatly 10
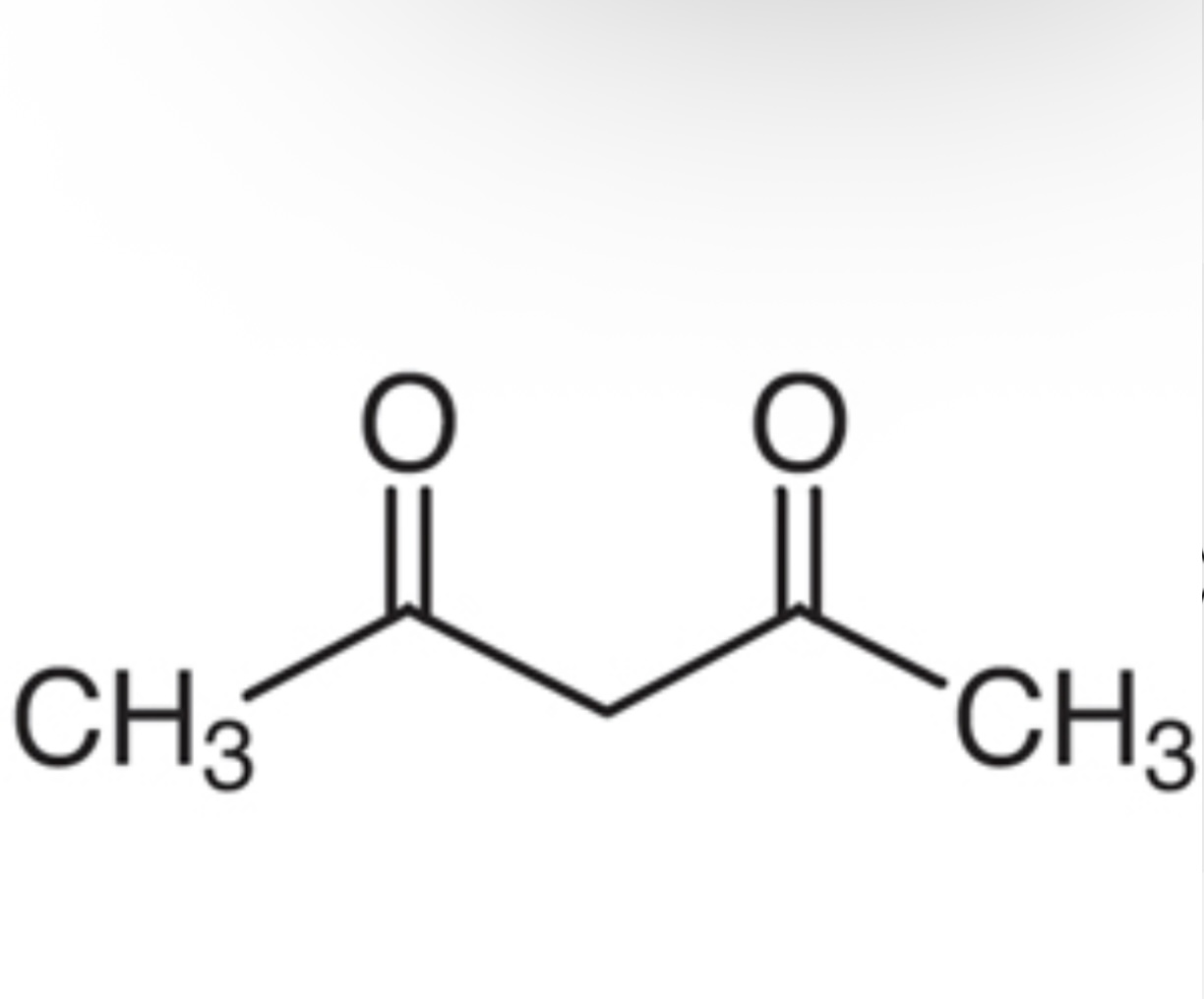
1,3-diketone pka?
pka is approximately 10
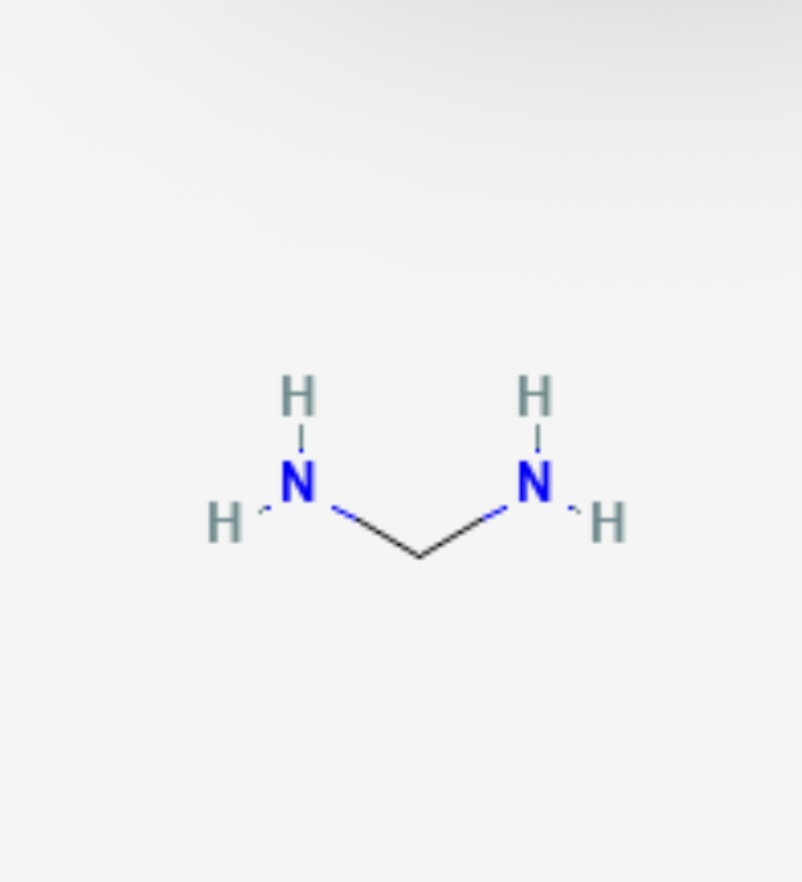
Diamine pka?
pka is approximately 10
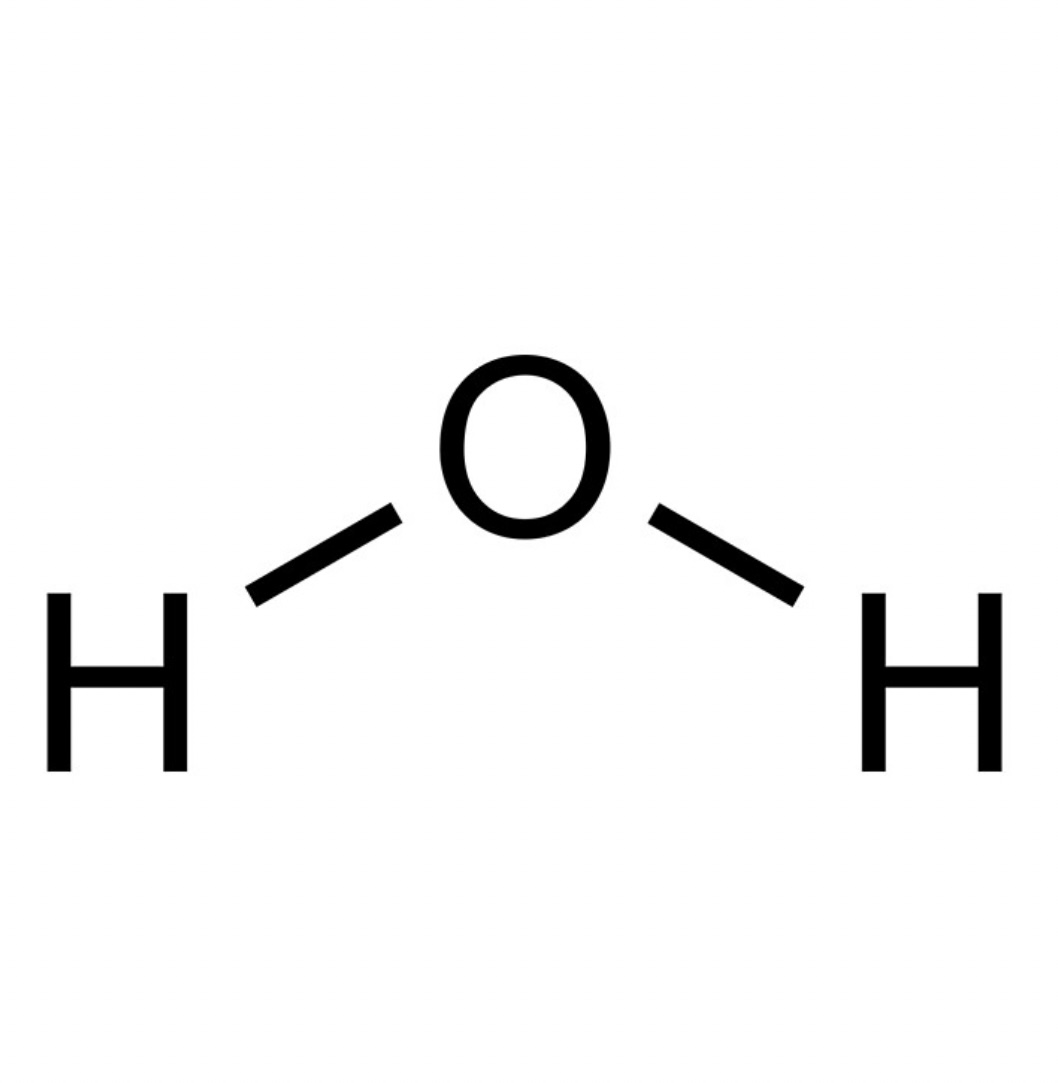
Water pka?
pka is approximately 14
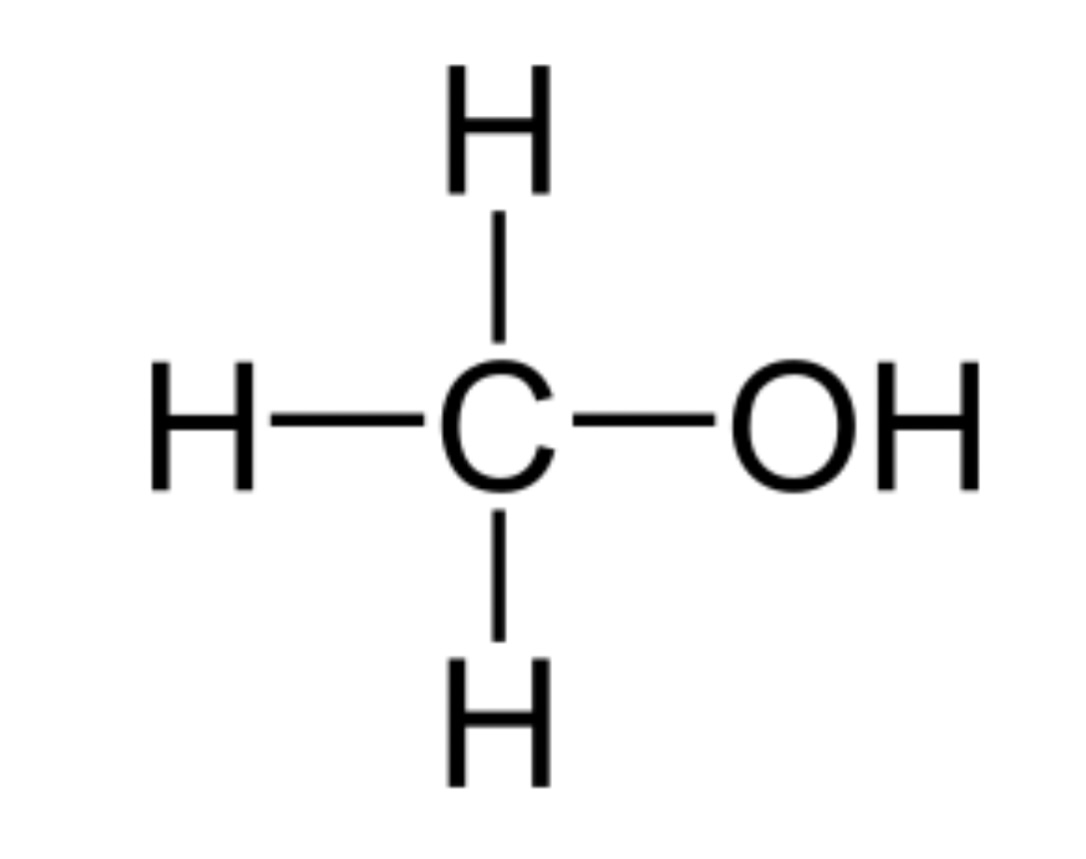
Alcohol pka?
pka is approximately 15-18
Primary alcohols are most acidic then secondary then tertiary
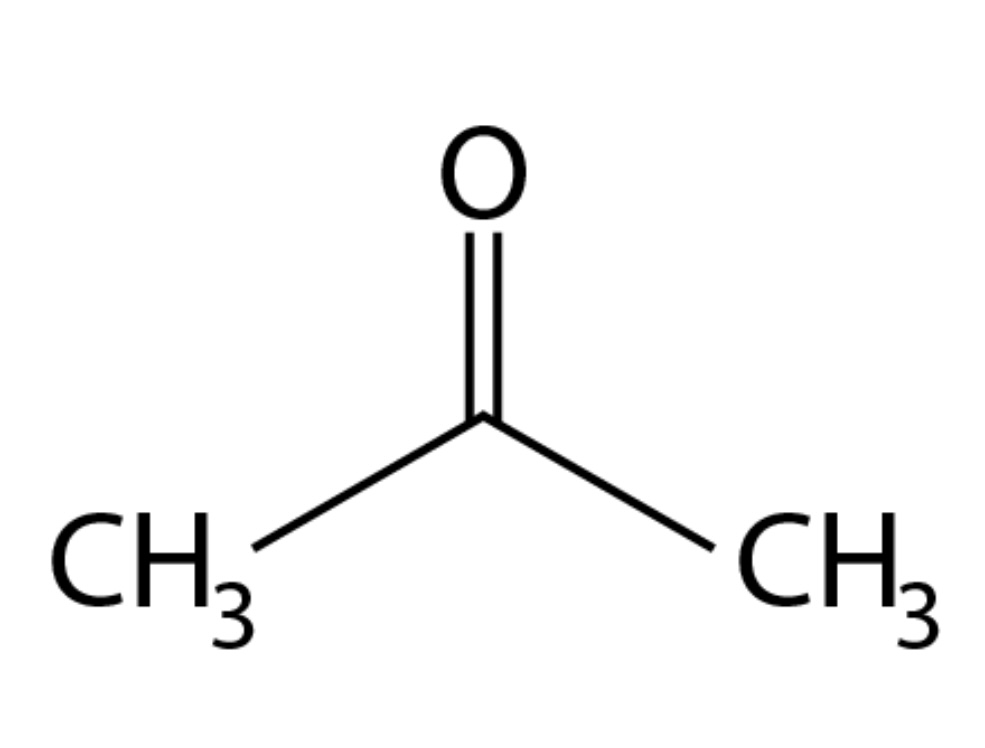
Ketone pka?
pka is approximately 20
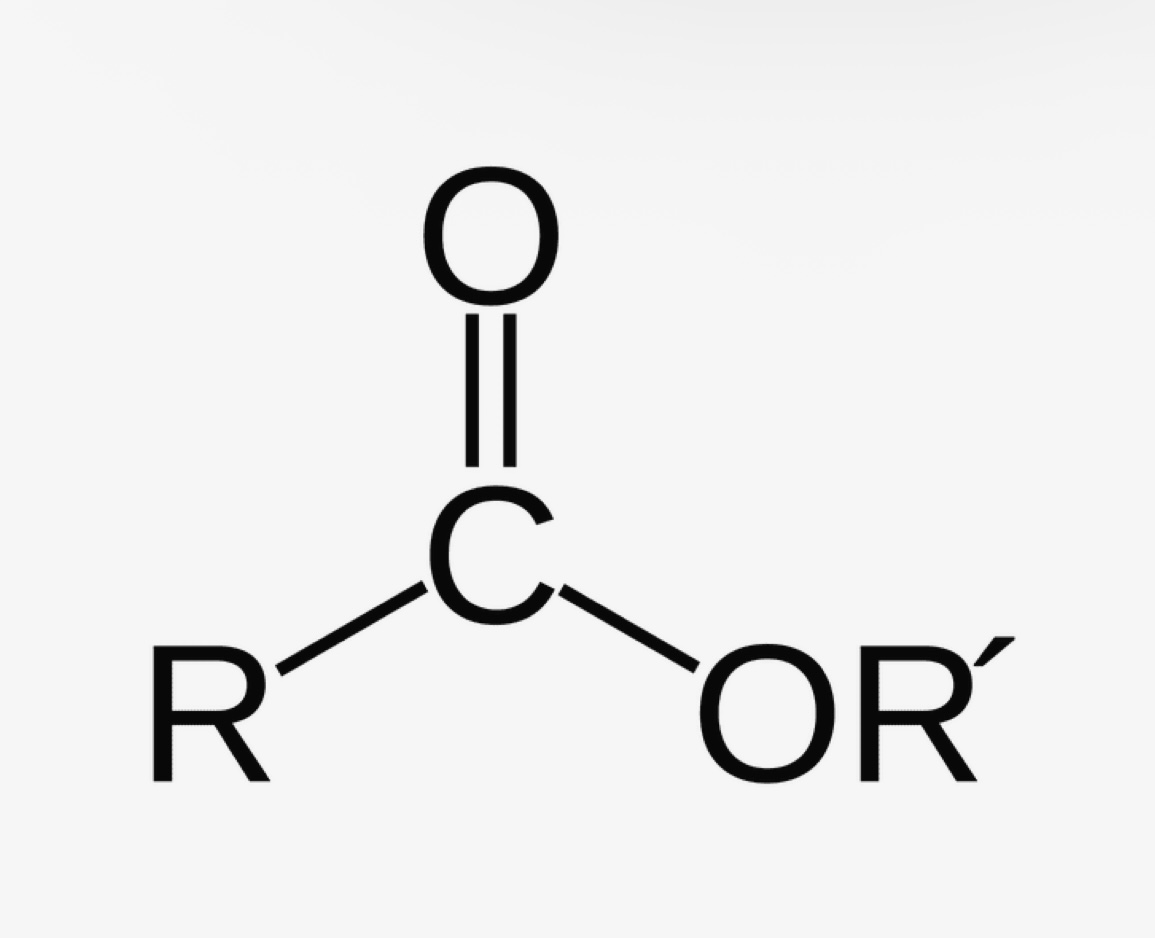
Ester (carbon next to ketone) pka?
pka is approximately 25
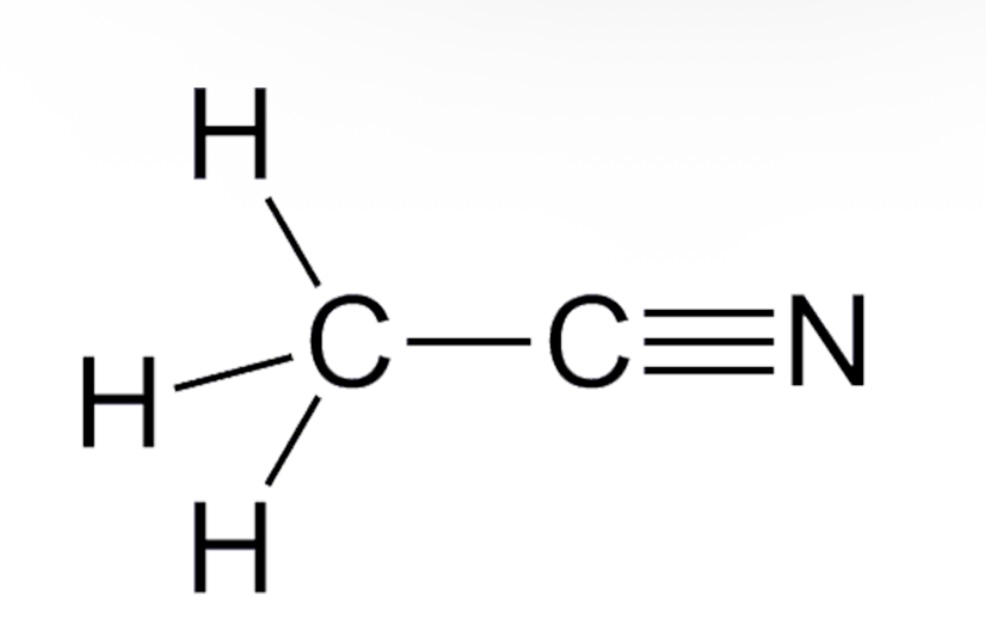
Acetonitrile pka?
pka is approximately 25
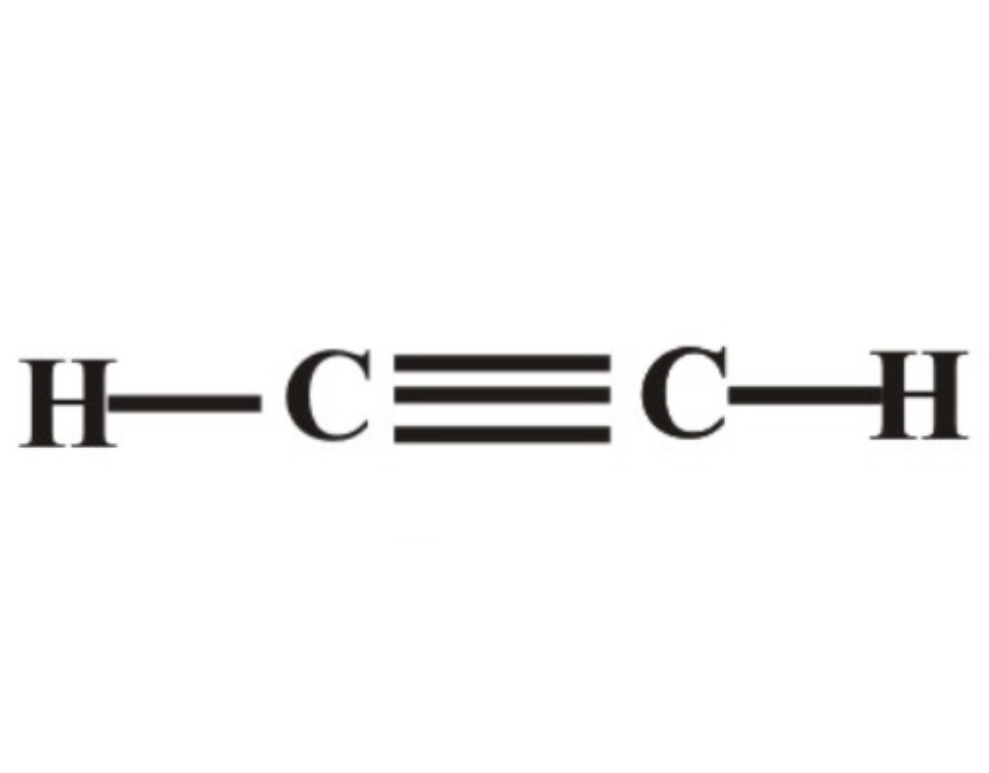
Alkyne pka?
pka is approximately 25
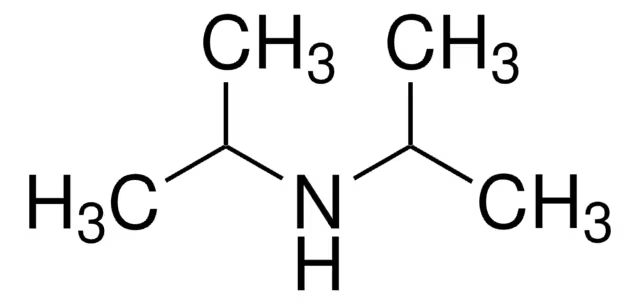
Isopropyl Amine pka?
pka is approximately 35
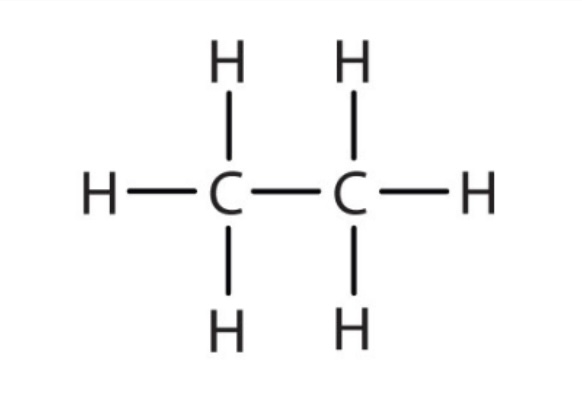
Alkane pka?
pka is approximately 60
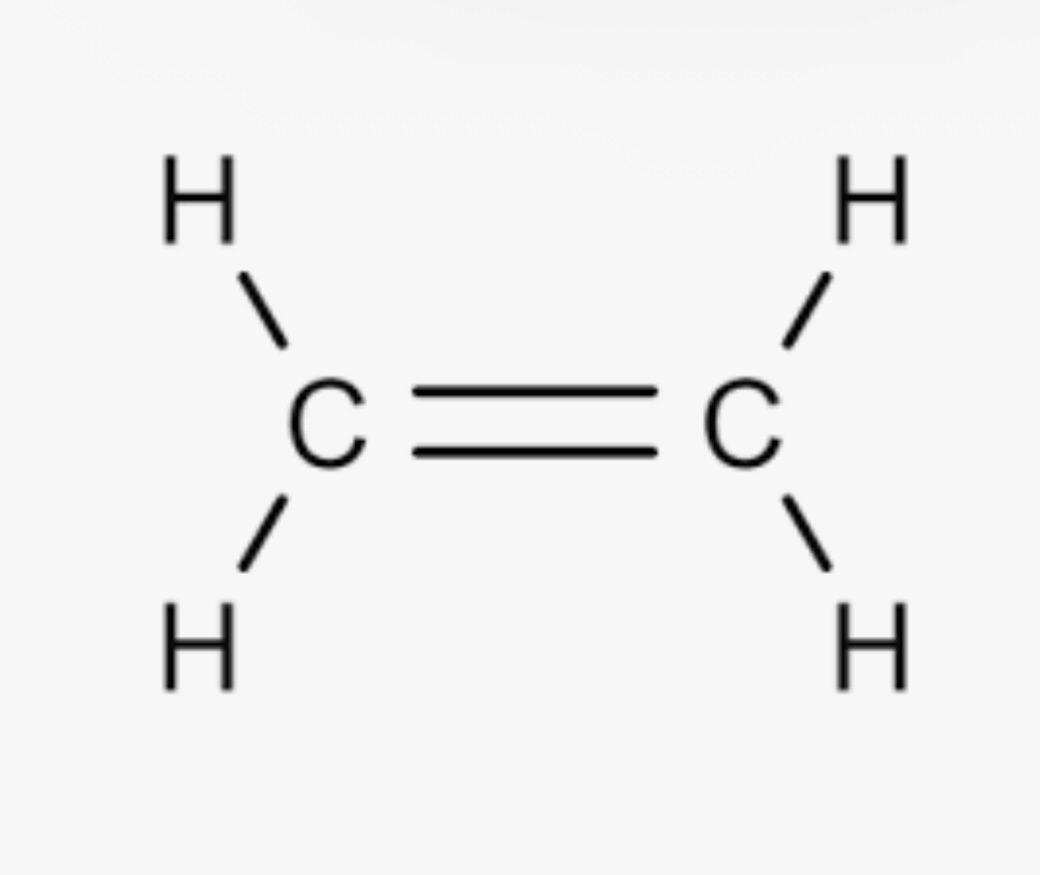
Alkene pka?
pka is approximately 44-45
Alkyne bond energy for C-H bond?
125 Kcal/mol
Alkene bond energy for C-H?
108 Kcal/mol
Alkane bond energy for C-H?
100 Kcal/mol
Allylic bond energy for C-H?
86 Kcal/mol
Alcohol bond energy for O-H?
110 Kcal/mol
Alkane bond energy for C-C?
82 Kcal/mol
Alkene bond energy for C=C?
82+64 = 146 Kcal/mol
Alkyne bond energy for C triple bond C?
82+64+64 = 210 Kcal/mol
Ether bond energy for C-O?
85 Kcal/mol
Keytone bond energy for C=O?
85+95 = 180 Kcal/mol
Peroxide bond energy for O-O?
30-50 Kcal/mol
Disulfide bond energy for S-S?
30-50 Kcal/mol
Rotational bond energy for a alkane bond (C-C)?
2.9 Kcal/mol
Rotational bond energy for an amide bond (C-N)?
17 Kcal/mol
If the energy is greater than __ an isomer will not interconvert at room temperature (change from one isomer to another)
greater than 22 Kcal/mol
Rank these carbonyl groups from acidic to basic:
Water
Aldehyde
Ester
Amide
Carboxylic Acid/Carboxylate ion
Keytone
Most Acidic Aldehyde > Keytone > Ester > Carboxylic Acid/Carboxylate ion > Water > Amide Most Basic
Lone pairs on the aldehyde carbonyl group make it a more acidic carbonyl than a carboxylic acid. Not looking at protons with this question strictly C=O acidity. Resonance stabilizes the charge through its other oxygen group for carboxylic acids making it more of a basic (stable) carbonyl group.
A value for methyl
1.70
A value for tert-butyl group
>4.5
A value for PbMe3
0.7, longer bond length so it does not have much of a diaxial interaction