4.3.2 Internal energy and energy transfers
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
What is internal energy?
Energy which is stored by particles within a system
What forms does internal energy take?
Kinetic energy and potential energy
How does heating increase the energy particles have?
It raises the temperature of the system or produces a change of state
What is specific heat capacity?
The amount of energy required to raise the temperature of 1kg of a substance by 1°C
What is the equation for specific heat capacity?
change in thermal energy = mass x specific heat capacity x temperature change
What is specific latent heat?
The amount of energy needed to change the state of 1kg of a substance without a change in temperature
What’s the equation for specific latent heat?
energy = mass x specific latent heat
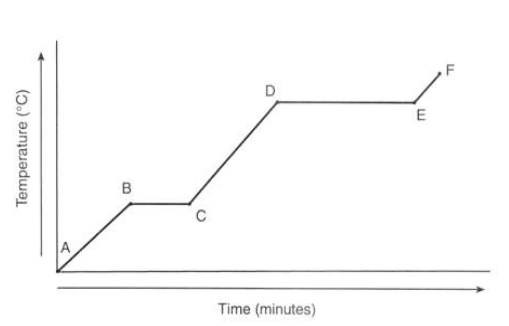
What is happening to the temperature of ice at point A?
It is currently a solid
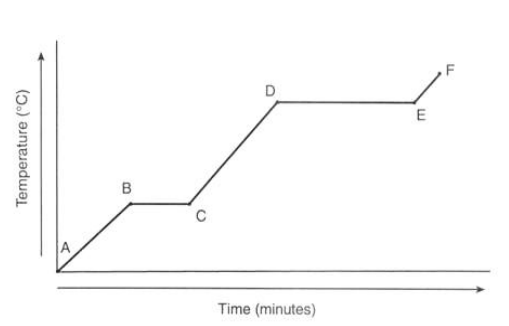
What is happening to the temperature at point B?
It reaches 0°C
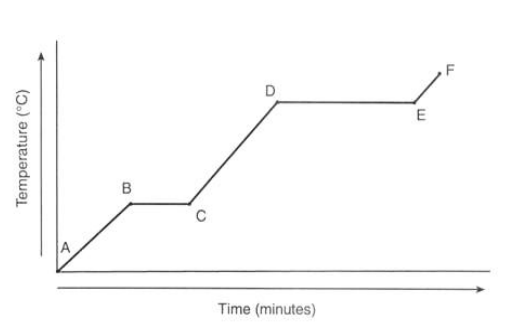
What is happening to the temperature from point B to C?
There’s no temperature change as the energy is used through melting
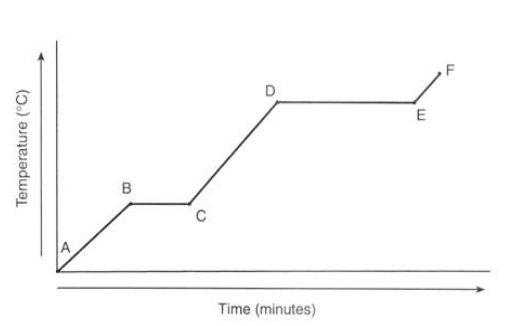
What is happening to the temperature from point C to D?
It’s in liquid state
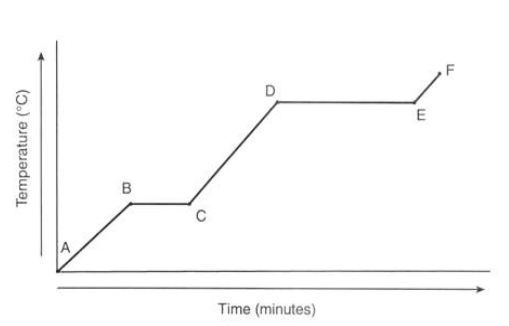
What is happening to the temperature from point D to E?
The water is boiling which takes longer as evaporation takes more energy
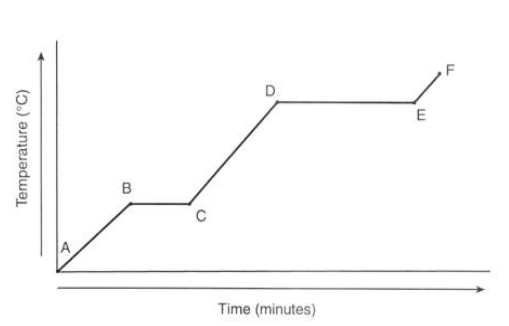
What is happening to the temperature from point E to F?
The gas is heating