MICR 270 mod 3 - the immune system
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
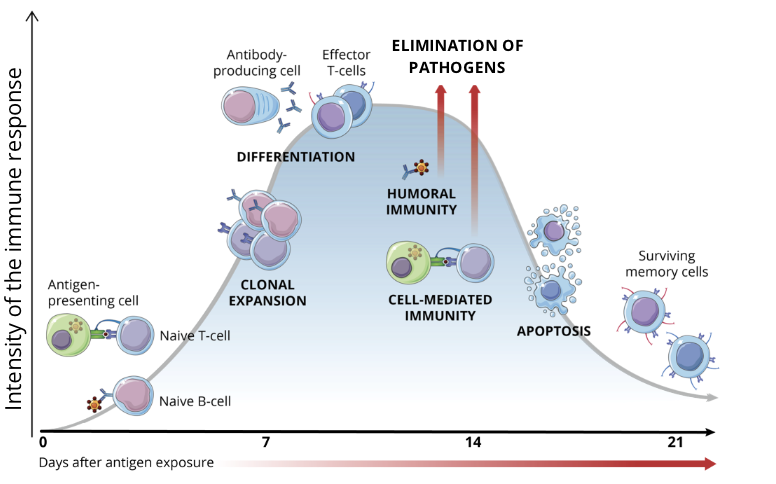
phases of the adaptive immune response
antigen recognition (antigen presentation)
lymphocyte activation
elimination of pathogens or non-self perceived antigens
apoptosis of immune cells (contraction)
establishment of immunological memory
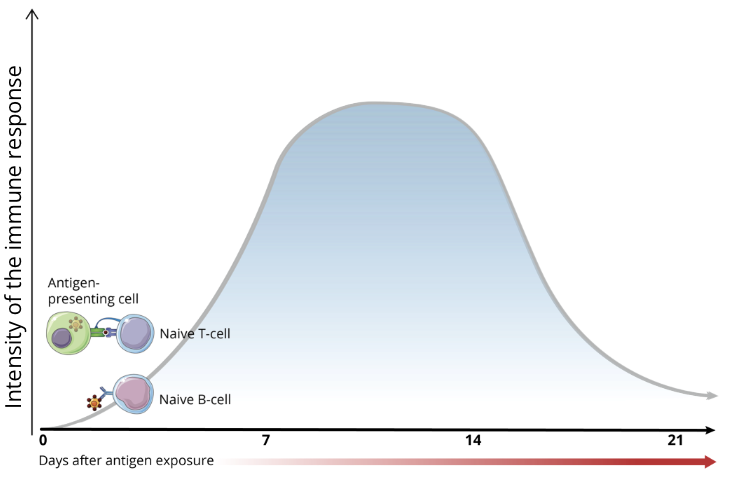
antigen recognition
after the pathogen has entered the body and evaded innate immune defences, PAMPs are seen by antigen-presenting cells (APCs), ie. dendritic cells and macrophages
APCs will present antigens to naive T cells via their surface Major Histocompatibility Complex (MHC) proteins
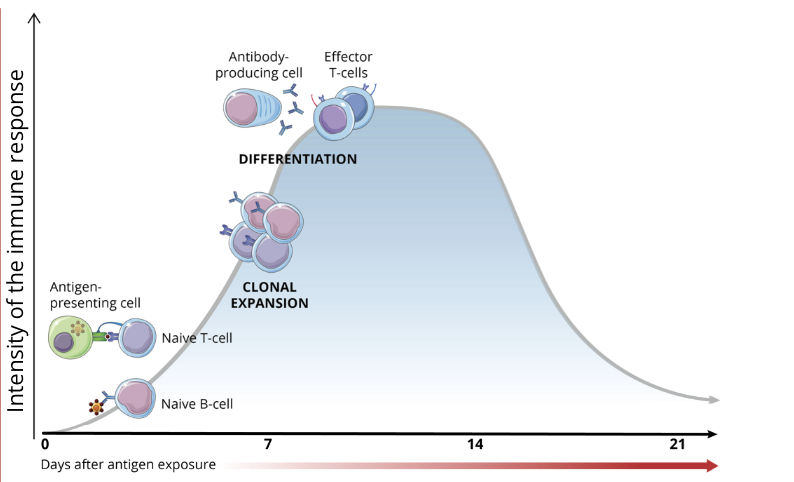
lymphocyte activation
the process of lymphocyte activation requires a series of cellular interactions (communication) which lead to T cell and B cell differentiation and clonal expansion (production of a large quantity of identical cells from the same original cell)
B cells → plasmocytes
T cells → helper T cells OR cytotoxic T cells
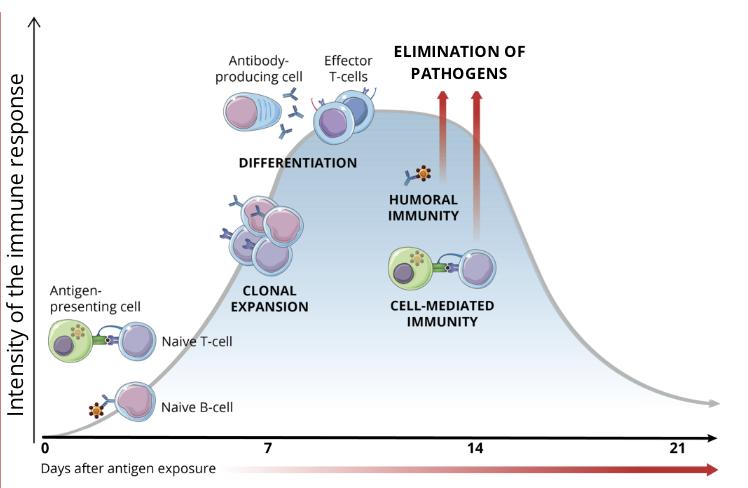
elimination of pathogens
depending on the type of pathogen invading the body, the most efficient defences are unleashed
Humoral Immunity
plasmocytes produce antibodies that bind to extracellular pathogens
Cell Mediated Immunity
cytotoxic T cells destroy infected intracellular pathogens or get activated by antigens presented by APCs
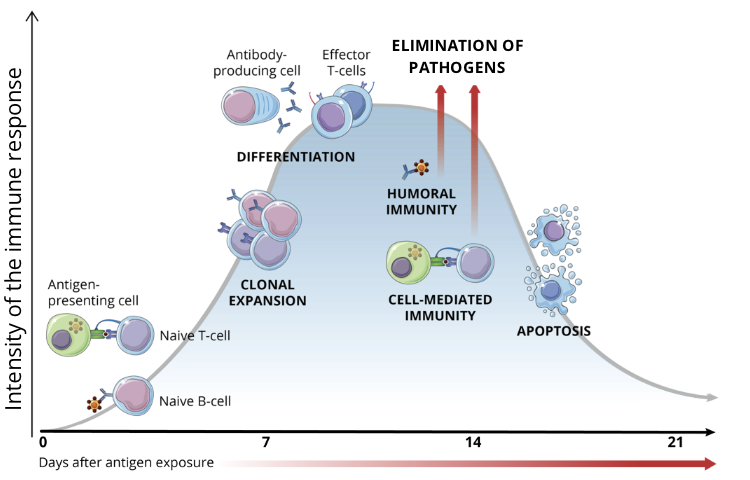
apoptosis of immune cells (contraction)
once the pathogen is eliminated, the vast majority of activated lymphocytes undergo apoptosis, and the immune response gradually declines
immunological memory
the few adaptive immune cells that survive the contraction phase differentiate into memory cells
when re exposed to the same antigen, these memory cells proliferate quickly to generate an immune response that is much faster and more robust than the first response to the pathogen
Differentiation between self and non self molecules
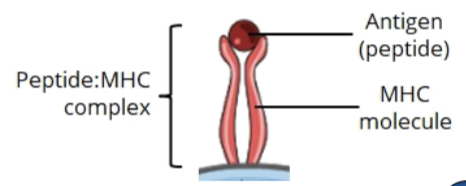
the Major Histocompatibility Complex (MHC) serves as a self-label and helps identify and recognize self from non self molecules to ensure the immune system does not attack the host
the role of MHC molecules
MHCs are molecules that display antigen specific peptides on the surface of cells
aThis MHC-antigen complex can be recognized by the TCR and its co-receptors (CD4 or CD8) to initiate an adaptive immune response, which leads to elimination of foreign antigens
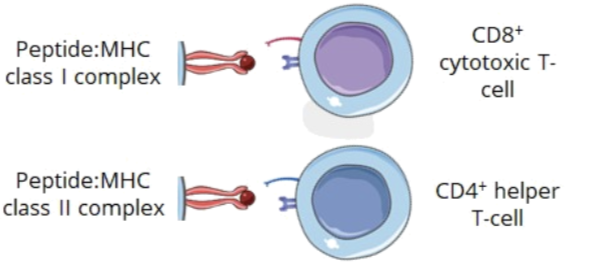
Classification of MHC molecules
there are 2 classes of MHCs:
MHC class I (CD8 cytotoxic T cell)
MHC class II (CD4 helper T cell)
these classes help to distinguish between different recognition patterns
Antigen Presenting Cells
helper T cells are essential in the process to induce an effective adaptive immune response
because T cells are not able to recognize extracellular pathogens by themselves, they require an intermediate to present them the antigens found inside the body
these intermediates are called antigen presenting cells (APCs)
APCs internalize pathogens by phagocytosis or receptor mediated endocytosis and process them into peptides
the peptides, also called antigens, are displayed on the MHC on the surface of the APC and can be recognized by T cells
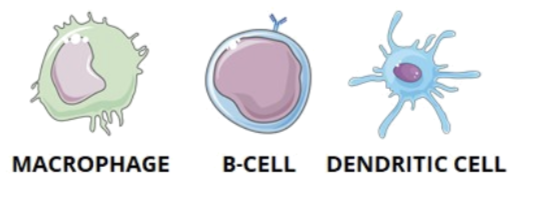
Professional APCs
they are the most efficient cells that both present antigens through MHC II and express costimulatory signals to activate helper T cells
macrophages
B cells
dendritic cells
Non professional APCs
Some other cell types can be induced to express MHC II complexes or stimulatory molecules, but normally they don’t
this is because these cells will rarely be needed in this specific function and only for short periods of time in case of a sustained inflammatory response
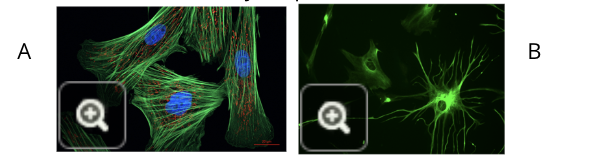
fibroblasts (image A)
glial cells (image B)
Antigen Processing
each antigen presented by an MHC molecule needs to be processed to for an effective peptide:MHC signalling complex
there are 2 pathways that lead to the formation of these surface complexes'
Endogenous Pathway
Forms peptide:MHC I complex, recognized by CD8 cytotoxic T cells
Exogenous Pathway
forms peptide:MHC II complex, recognized by CD4 helper T cells
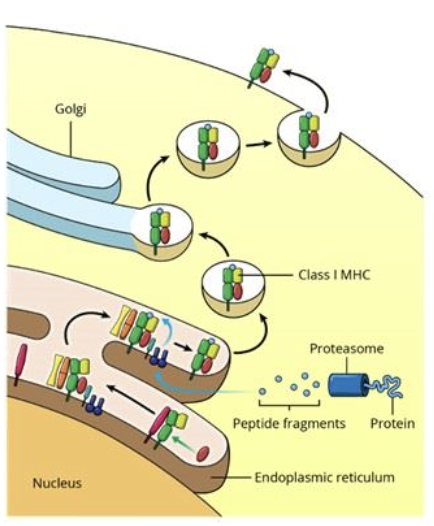
Antigen Processing by Endogenous Pathways
the endogenous pathway allows the cell to process self or foreign intracellular particles and present them at the cell surface in order to be recognized by T cell receptors on cytotoxic T cells
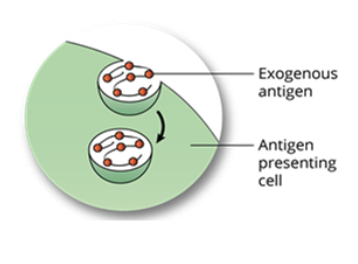
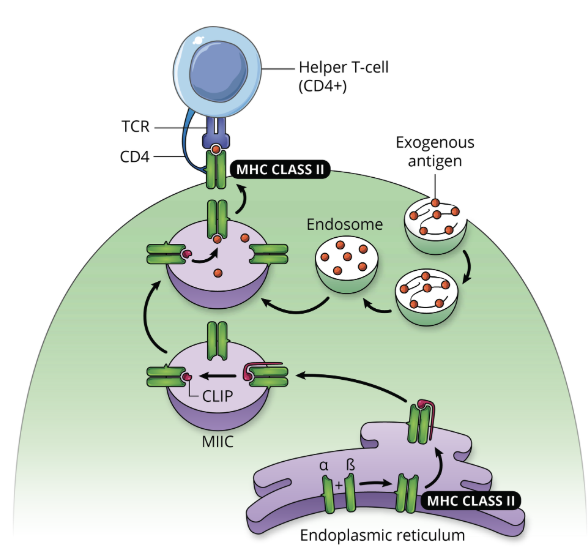
Antigen Processing by the Exogenous Pathway: step 1, antigen engulfment
antigen presenting cells ie. macrophages, B cells, or dendritic cells, engulf the foreign antigen by endocytosis forming an endosome
the antigen is generally recognized by PRRs (pattern recognition receptors)
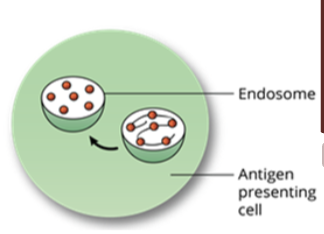
Antigen Processing by the Exogenous Pathway: step 2, proteolytic processing
foreign antigens inside the endosome are broken down into fragments by proteolytic processing
protease cleaves one or more bonds in a target protein to modify its activity (activation, inhibition, or destruction)
Antigen Processing by the Exogenous Pathway: step 3, formation of MHC-antigen complex
the vesicle containing the foreign fragments fuses with vesicles containing MHC molecules (originating from the endoplasmic reticulum via the Golgi), forming MHC-antigen complexes
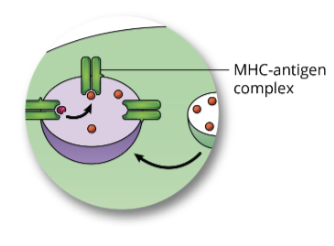
Antigen Processing by the Exogenous Pathway: step 4, cell surface expression
the MHC-antigen complex is transported to the plasma membrane, were it will be displayed on the surface of the cell
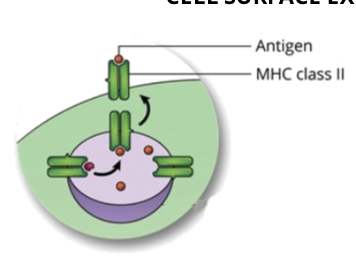
Antigen Processing by the Exogenous Pathway: step 5, recognition by helper T cell
the T cell receptor (TCR) on the surface of a helper T cell binds to the MHC-antigen complex on the cell surface of the APC, which will initiate an adaptive immune response
overview of antigen processing by the exogenous pathway (graphic)
B cell & T cell receptors
the adaptive immune system is specific to an antigen and B cells and T cells are the main components of the adaptive immune system
in order to initiate an immune response, antigens must be recognized by receptors on the surface of these cells
B cells and T cells each express specific receptors called BCR and TCR respectively
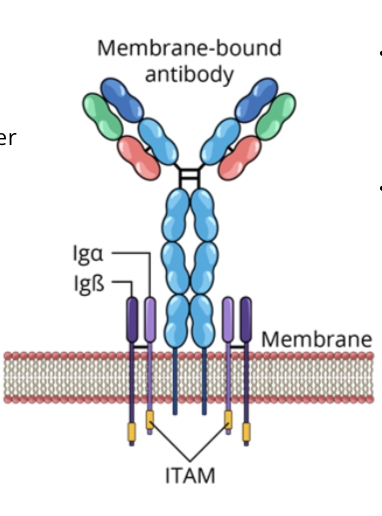
B cell receptors (BCR)
the BCR is composed of a membrane bound antibody and signal transduction molecules (ITAMs)
An immunoreceptor tyrosine based activation motif (ITAM) is composed of a repeated sequence of 4 amino acids in the cytoplasmic tails of cell surface proteins
BCRs recognize and bind to extracellular pathogens or toxins directly
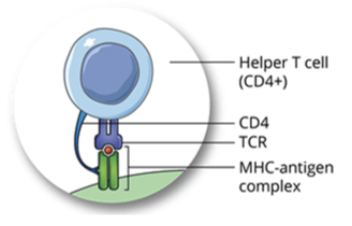
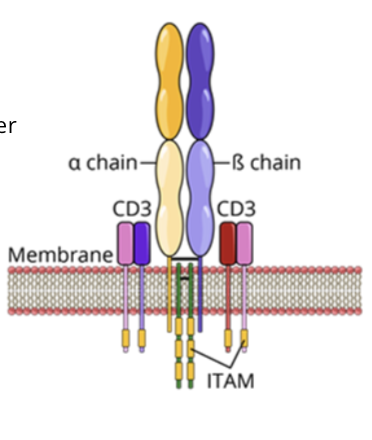
T cell receptors (TCR)
TCR complex is formed of a membrane bound antigen specific molecule and signal transduction molecules (CD3 and ITAMs)
TCRs in association with a coreceptor (CD4 or CD8) recognize and bind to peptide:MHC complex
lymphocyte activation
lymphocyte activation includes many interactions with other immune cells, which will mediate the efficiency of the specific immune response
Cytokine networks
cytokine networks coordinate appropriate immune responses and modulate the balance between humoral and cell mediated immunity
cytokines are divided into various classes depending on their functions and the structure of their receptor:
chemokines
interleukines
interferons
tumor necrosis factor
growth factors
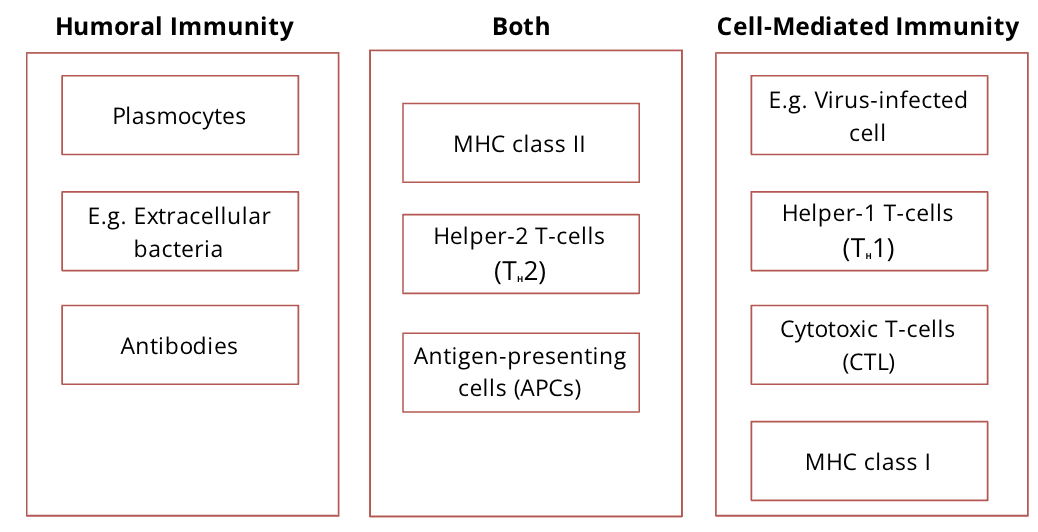
Components of humoral vs cell mediated immunity
T cell dependent B cell activation
the immune system requires the assistance of CD4 helper T cells to stimulate humoral immunity and the differentiation of B cells
Upon activation, B cells can differentiate into antibody producing cells
T cell dependent activation of B cells is an intricate process that involves specific signals essential to the production of functional plasmocytes and memory B cells
the interactions between a T cell and a B cell induces the exchange of activation signals between the two lymphocytes, allowing the activation of strong and efficient humoral immune response
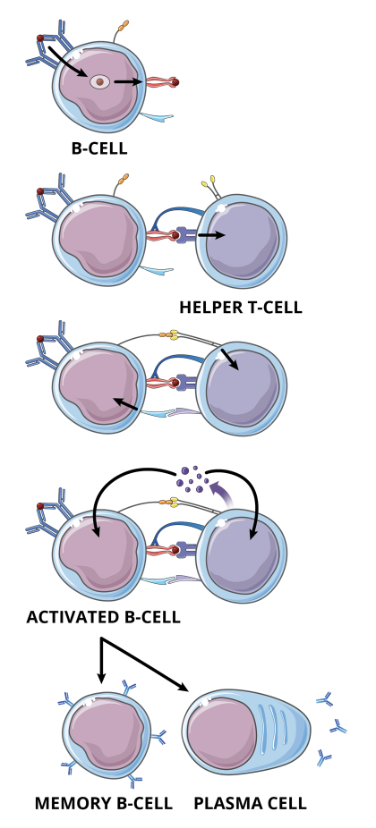
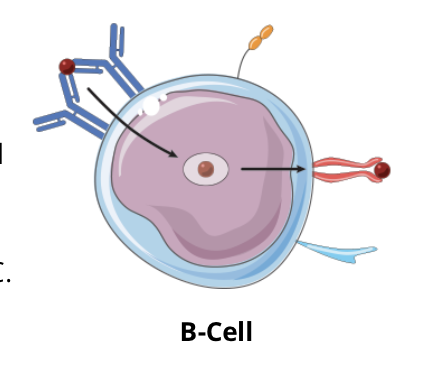
T cell dependent B cell activation signalling pathway: step 1, Peptide-MHC II complex
as the antigen (red circle) binding to the BCR on a specific B cell does not produce a strong enough signal to activate the cell, the antigen is internalized by receptor mediated endocytosis, processed, and displayed on the cell membrane in the context of MHC
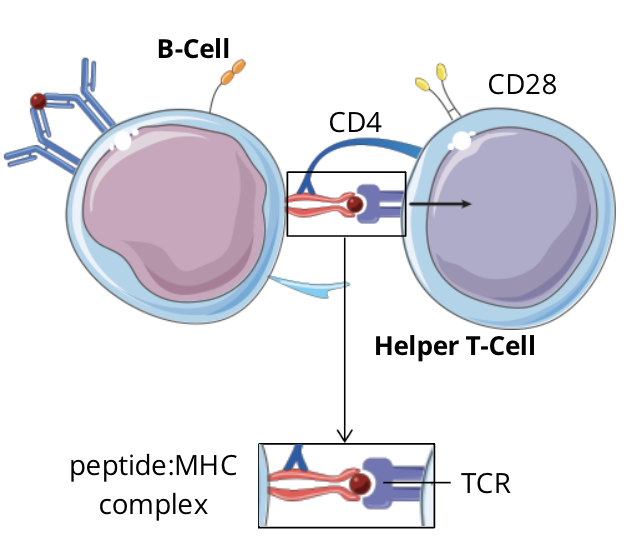
T cell dependent B cell activation signalling pathway: step 2 - signal 1, TCR-peptide:MHC complex
the specific TCR complex and CD4 coreceptor on the T cell recognizes and binds to the peptide:MHC II complex expressed on the B cell
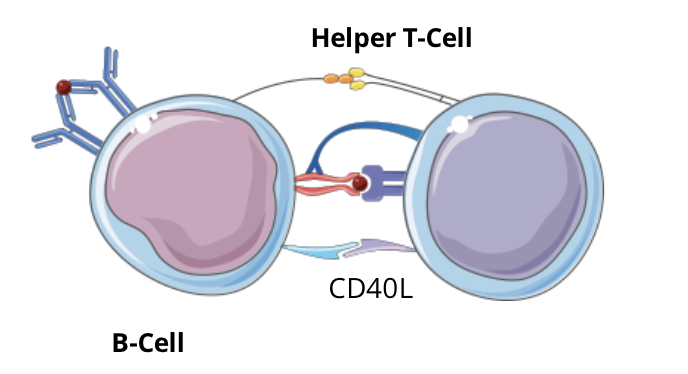
T cell dependent B cell activation signalling pathway: step 3, expression of costimulatory molecules
signal 1 (TCR-peptide:MHC complex II) induces the expression of CD40L on the cell surface of the helper T cell
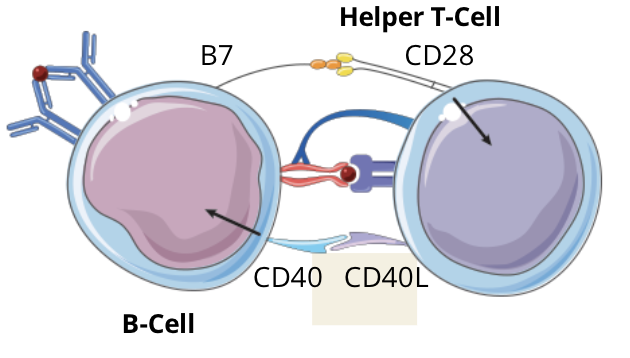
T cell dependent B cell activation signalling pathway: step 4 - signal 2, Co-Stimulation
CD40L and CD28 expressed on the T cell, respectively, bind to CD40 and B7 expressed on the B cell, inducing a costimulatory signal in both cells
T cell dependent B cell activation signalling pathway: step 5 - signal 3, cytokines
the activated helper T cell secretes cytokines which will bind to their associated cytokine receptor located on both cells (a paracrine and an autocrine effect)
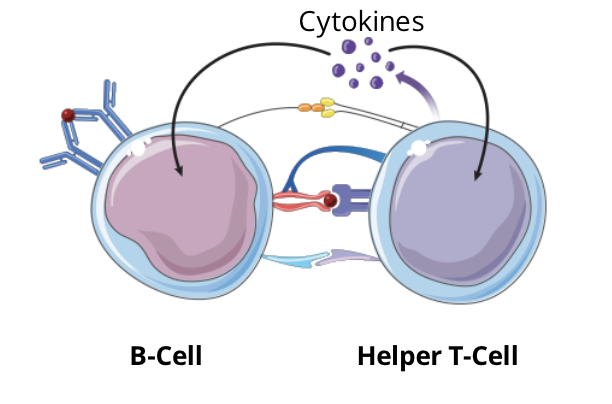
T cell dependent B cell activation signalling pathway: step 6 - outcome
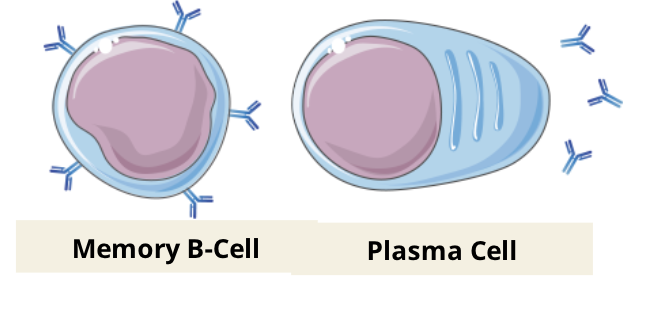
the combination of these three signals ultimately stimulate the proliferation and differentiation of B cells, forming the humoral immune response against the specific antigen
The immune synapse structure
the interaction between a T cell and an APC will form a structure called an immune synapse
T cells will not become properly activated if only signal molecules such as a T cell receptor and a peptide:MHC complex interact
an immune system synapse consisting of signal molecules and adhesions proteins must be formed
the immune synapse is often arranged into a bullseye pattern with three rings depicting three different cell clusters with similar functions, called supra molecular activating clusters (SMACs)
However, the immune synapse between T cells and other APCs can be arranged into different patterns with similar components
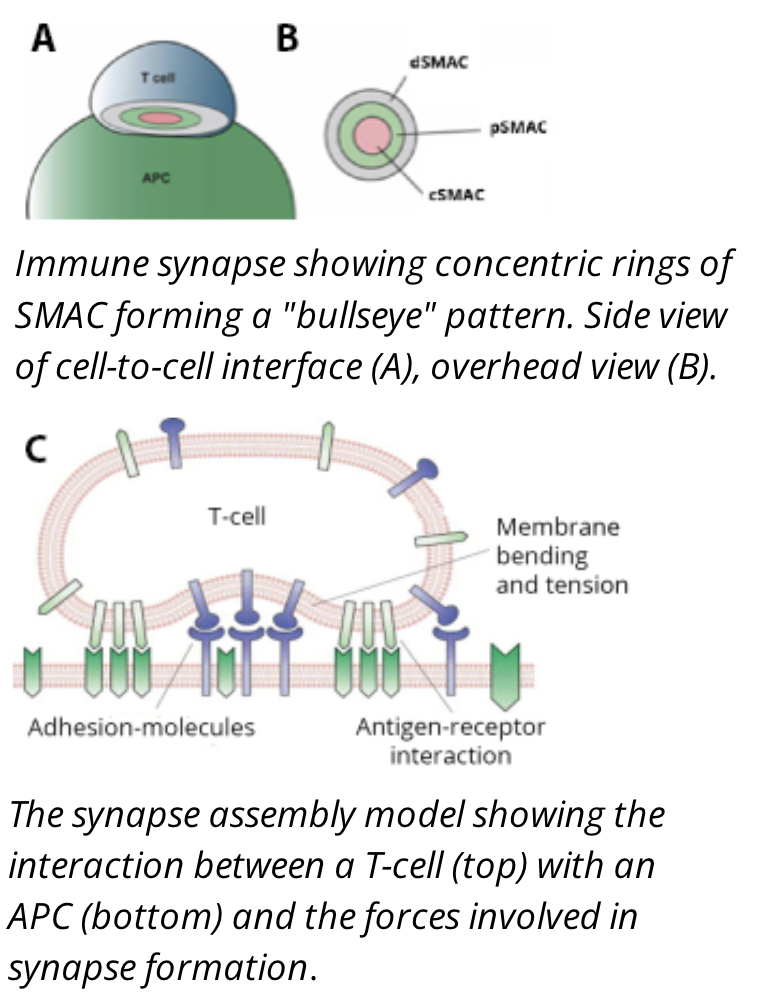
Signal Molecules (cSMACs)
the central SMAC (cSMAC) contains the molecules responsible for signalling between the two cells such as the TCR and Peptide:MHC molecules
Adhesion molecules (pSMACs)
the peripheral SMAC (pSMAC) contains adhesion proteins, such as integrins and cytoskeletal linker proteins, responsible for keeping the cells in contact long enough for signals to propogate
signal regulation molecules (dSMACs)
the distal SMAC (dSMAC) consists of proteins with large extracellular domains that are responsible for helping regulate signal transduction
Immune synapse function
the formation of an immune synapse serves several important functions:
the primary goal of the immune synapse is the effective activation of the T cell
the synapse holds signal proteins together to form stronger connections, which give enough time for the right amount of signals to be produced
the synapse leads to the reorganization of structures inside the T cell, directing the release of cytokines close to the target cell
the synapse regulates lymphocyte activation
Cytokine networks
cytokines are chemical messengers secreted by immune cells that play a key role in cell to cell communication
the function of cytokine signalling is to regulate immune processes, such as immune responses
classification of cytokines can differ depending on which criteria they are organized (will be classified based on function for this course)
Chemokines
induce chemotaxis (movement of an immune cell in the direction of an elevated concentration of chemoattractant molecules, ie. chemokines)
call in cells to the region of infection or injury
play a key role in:
inflammation; wound healing
cell mediated and humoral immune responses
hematopoiesis
Interleukines (IL)
contain over 10 subfamilies
regulate immune and inflammatory responses
primarily affect the proliferation and differentiation of various hematopoietic and immune cells
Interferons (IFN)
induce an antiviral state (inhibit the replication process of viruses)
help regulate immune responses
tumor necrosis factor (TNF)
involved in systematic inflammation ie. septic shock (a serious medical condition associated with a significant drop in blood pressure that can lead to respiratory or heart failure, and death)
involved in tumor regression
can cause apoptosis
Growth factors
stimulate:
growth
proliferation
healing
cellular differentiation
regulate a variety of cellular processes such as immune responses
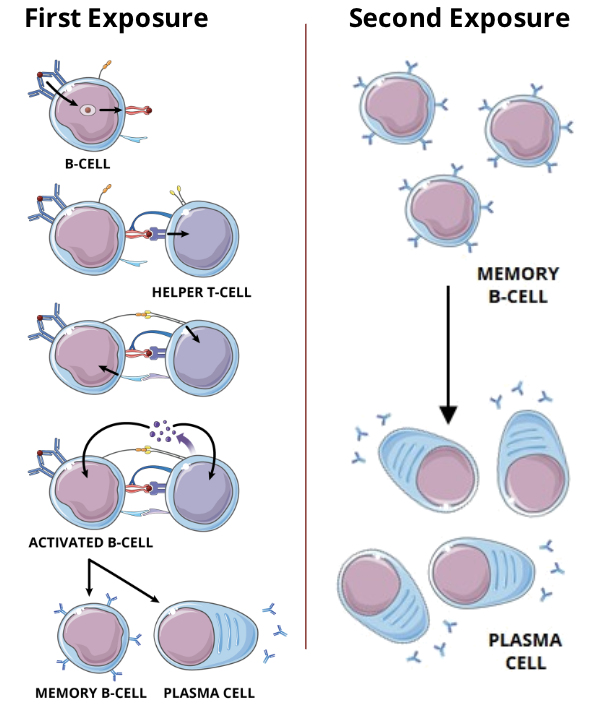
Memory B cells
immunological memory occurs when there is a second encounter with an antigen, that induces a heightened state of immune reactivity
this is mediated by memory B cells
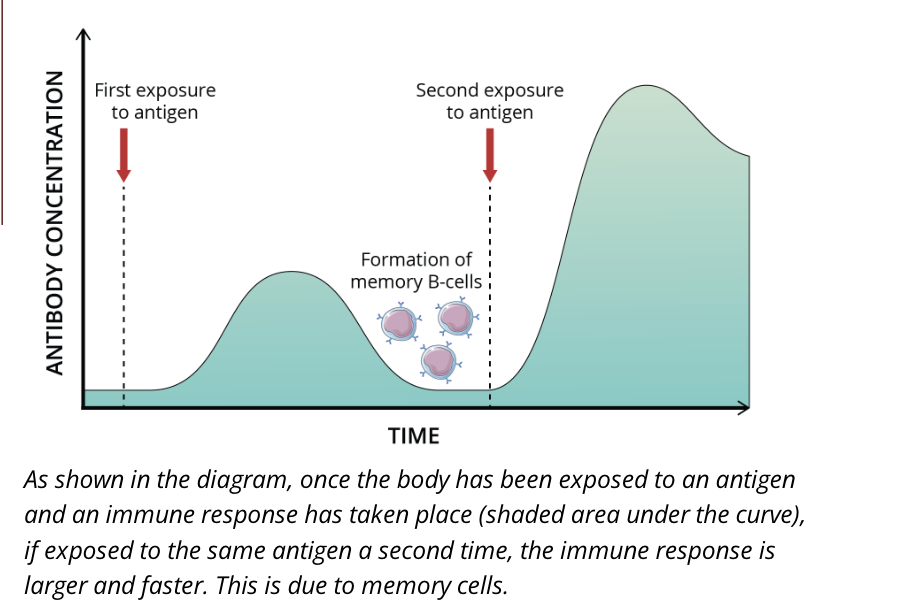
Second exposure to an antigen
memory B cells differentiate from naive B cells and display the same membrane bound antibody as their parent cell
these cells have a longer lifespan than naive B cells, which contributes to lifelong immunity against many pathogens
individuals can often only become sick from a specific strain of bacteria for example, once in their lifetime
this is because when a person becomes re-infected, the immune system is so efficient that they never display symptoms
Immunological memory
generation of memory cells generates immunological memory, which is the most efficient long term protection against known pathogens
there are different ways to get immunized against a pathogen, through natural and artificial means induced in an active or passive fashion
however, some of these immunization pathways never result in the development of immunological memory
natural passive immunity
acquired by the fetus or newborn from the mother
placental transfer of antibodies during pregnancy or breastfeeding
short lived immunity, approx 6 months
no immunological memory for the recipient
artificial passive immunity
acquired by injection of serum containing antibodies
immunity is temporary
no immunological memory for the recipient
natural active immunity
acquired through infection by a pathogen, possibly leading to symptoms/a disease state
development of innate and adaptive immune responses
immunological memory has a significant chance of being developed
Artificial active immunity
acquired through vaccination
development of innate and adaptive immune responses
normally, no symptoms/disease states are present
immunological memory has a significant chance of being developed