Colloids and suspensions
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
88 Terms
What is a dispersed system? How is it different to a solution?
-a system in which one component is dispersed as particles or droplets throughout another component. 2 phase system
-solution=1 phase system i.e. only one phase present. The molecules are dispersed on the molecular scale. Each molecule is isolated from all other drug molecules
What are colloidal suspensions?
-dispersions in which the size of the dispersed particles in the continuous phase is in the range 10-9 -10-6 m (less than 1um)
What is a particle?
-aggregates consisting of many drug molecules
What is the particle size in a suspension?
generally more than 1um
Give 4 reasons why we use suspensions
-poorly soluble drugs can’t always be made into solutions
-for taste making (may be less noticeable taste in suspensions)
-the drug may be more stable if formulated as a suspension rather than a solution
-the drug may be more stable as a solid, so we make a suspension just before dispensing
Give examples of a colloidal suspension
calamine lotion
amoxicillin suspension
State the 4 features that make a good suspension and why
the suspension must be easy to disperse upon shaking and easy to re-disperse (settling down is fine as long as it is easy to re-disperse)
the suspension should contain particles which are small and the same size (for dose uniformity). This is to ensure that the pts don’t find the suspension gritty
the suspension must be as homogenous as possible. The re-dispersion should not last a very short period of time. The particles should be evenly distributed after shaking to ensure same dose given each time.
high puritability
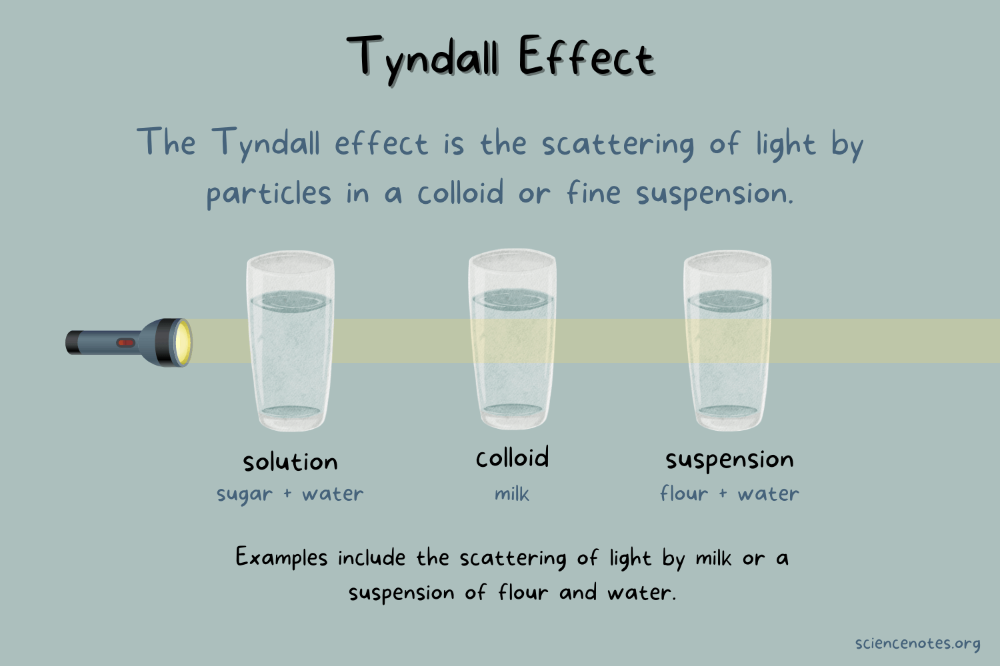
Explain the Tyndall effect
If a beam of light passes through a true solution, there is very little scattering of light, so the beam of light can’t be seen
-in a colloid, the particles scatter the beam of light, so you can see the path.
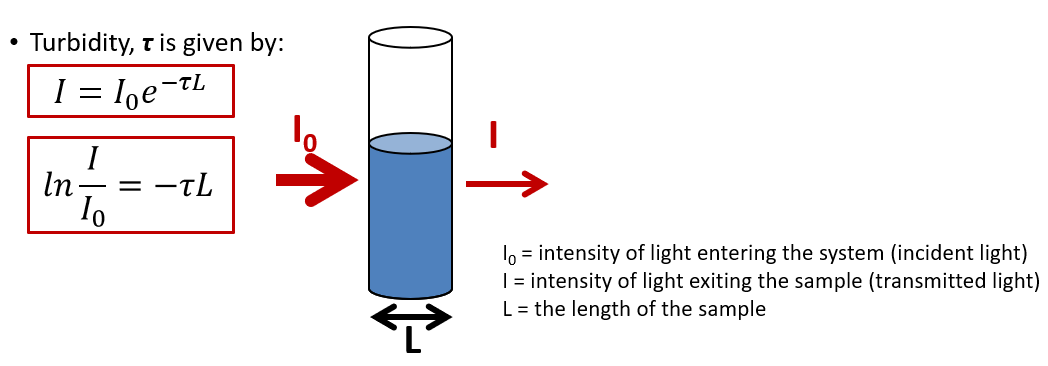
What is turbidity (as a quantity) and what is the equation for it?
How much solid you have in a suspension.
The less light that passes through the sample, the more turbid it is – and the greater the concentration of the dispersed phase
Explain motion in colloids
kinetic property
brownian motion=random motion of particles in liquid or gas
the particles in a colloid undergo Brownian motion because they are small. This means there will be random movement and collisions of the dispersed particles throughout the continuous phase.
Random collisions with: solvent molecules, other particles and container wall
-The collisions between particles may cause repulsive forces, especially if the particles have like charges or are interacting through certain physical forces (like electrostatic repulsion in the case of charged particles). This repulsion prevents the particles from clumping together and maintains a degree of separation between them.
brownian motion—→collisions—→repulsion—→stable dispersion
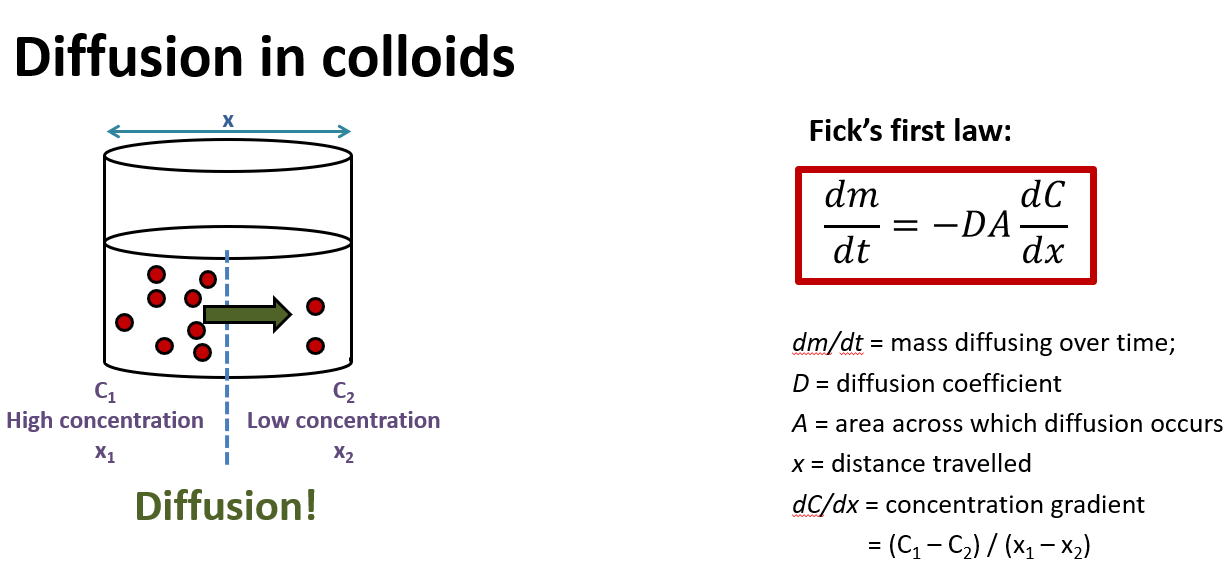
What is Fick’s law used to calculate?
used for diffusion (in colloids and any type of diffusion)
What is fick’s law equation?
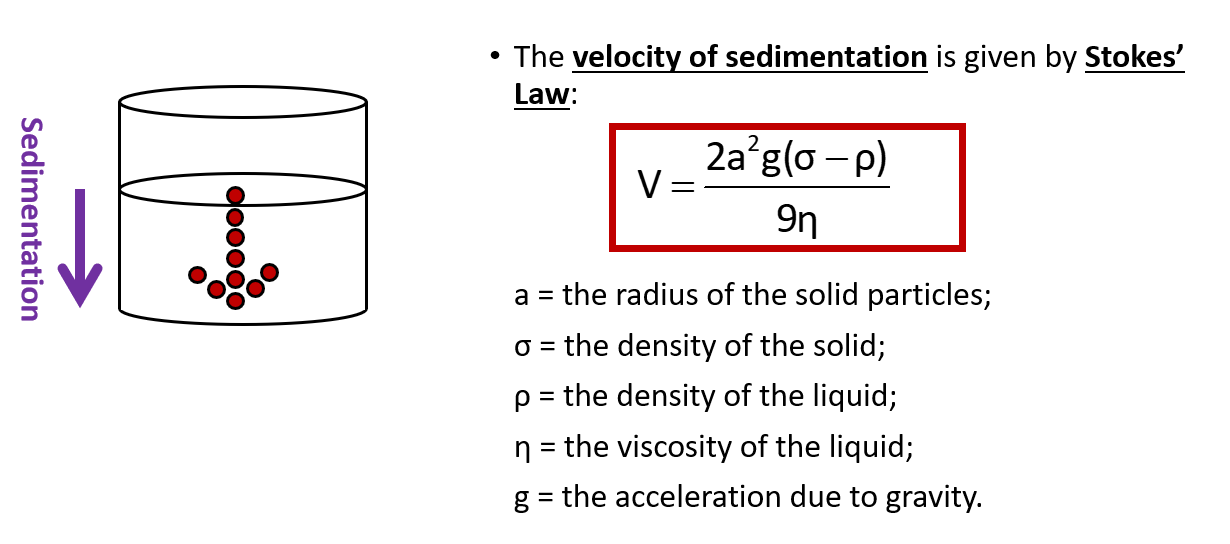
What is the equation for calculating velocity of sedimentation?
Stoke’s law
stoke’s law is for…
suspensions and emulsions
calculating velocity of sedimentation
Does increasing the radii of the particles increase or decrease sedimentation velocity? Why?
-increase
-because ‘a’ from stoke’s law equation gets smaller
Does increasing the density of the solid increase or decrease sedimentation velocity? Why?
increase
because σ will get larger, so will sedimentation velocity
Does increasing the density of the liquid increase or decrease sedimentation velocity? Why?
-decreases it
ρ will go down
Does increasing the viscosity of the liquid increase or decrease sedimentation velocity? Why?
decrease
-because η will go up
Does taking the suspension to the moon increase or decrease sedimentation velocity?
decreases because gravity on moon is weaker so g would be lower
If the system cakes, is it reversible/redispersible?
no, so suspension not achievable.
How do you calculate sedimentation ratio?
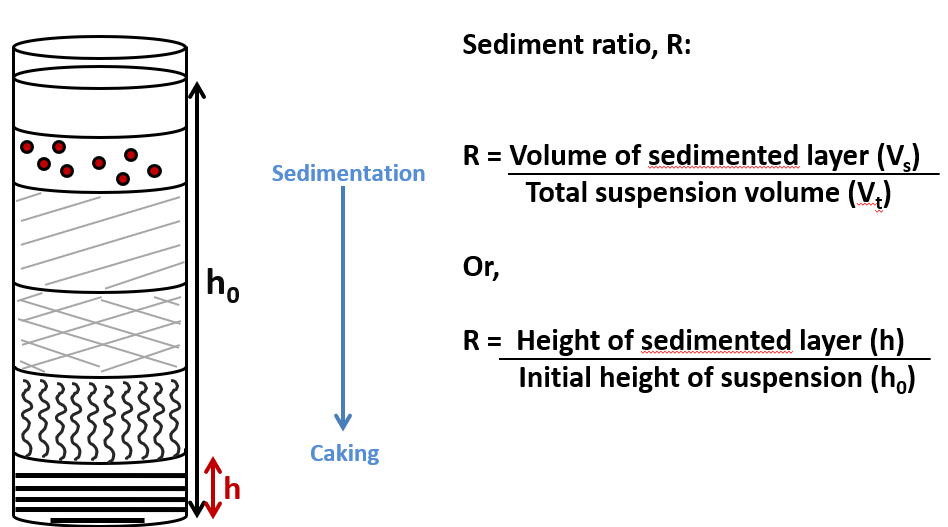
How would the sedimentation ratio vs time graph look like for flocculated system vs deflocculated? Explain the graph
Doesn’t reach zero because the particles take up some volume/height in the container
d for deeper
-In deflocculated system, sedimentation occurs slowly as there aren’t aggregates, so less density as it is individual particles that are settling. Therefore, sedimentation ratio gradually decreases over time and levels off at a lower value, indicating minimal liquid trapped in the sediment
-in flocculated system, sedimentation happens quicker because flocs are larger and settle quicker as a result. The sediment ratio levels off sooner and at a higher value, indicating lots of liquid is trapped within the flocs. This results in a larger sediment volume compared to the deflocculated system, so higher sediment ratio
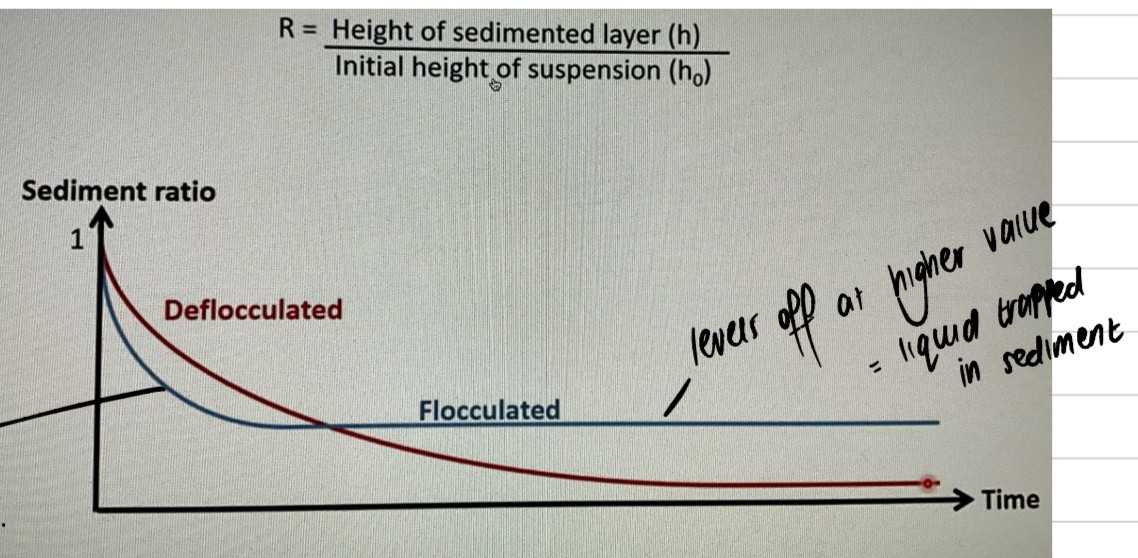
How would the sedimentation ratio vs time graph look like when there’s a viscosity enhancer vs no viscosity enhancer?
straight flat blue line=caking
How are suspensions made?
-the drug must be small particles of uniform size
-if the drug is water-insoluble, a wetting agent (which is a type of surfactant) should be added. This breaks the interfacial tension, ensuring that solid particles disperse easily throughout the liquid, which reduces clumping as there is decreased surface tension
What does a wetting agent do?
-This breaks the interfacial tension, ensuring that solid particles disperse easily throughout the liquid, which reduces clumping as there is decreased surface tension
-they decrease adsorption of particles to the container by applying a repellent coating to the particles in the suspension. Without the wetting agent, the hydrophobic particles would cling onto the container to get away from solvent. This allows for dispersion
What is interfacial tension?
-an energy barrier which prevents the liquid spreading around the solid.
What does low interfacial tension mean?
the liquid spreads around the particle so give it hydrophilicity. This makes a good suspension
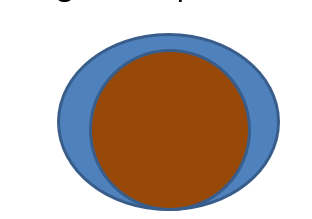
What does high interfacial tension mean?
: the liquid does not spread around the particle. This will give a bad suspension
State 3 types of wetting agents
-surfactants
-hydrophilic colloids
-simple solvents
What is a deflocculated system?
-particles are far apart from each other
What is a flocculated system?
-aggregates of particles with the solvent trapped in the aggregates. can have sedimentation of course, but easy to redisperse. Partially de-flocculated system is the BEST for suspensions
Why does a deflocculated system eventually lead to caking?
-slower rate of settling than in flocs, so prevents liquid entrapment in the sediment. Densely pack at the bottom over time, leading to caking and very difficult to redisperse
Why is liquid entrapment in flocs good?
easy redispersion
State the differences between a flocculated and deflocculated system (6)
-in floc, there’s loose aggregates of particles but in de, the particles exist as discrete units
-in floc, there is a large volume of final sediment but in de, there’s a small volume
-in floc, there’s a rapid sedimentation rate but in de, there’s a slow
in floc, suspension clears quickly but in de, it remains cloudy for a prolonged time
-in floc, the liquid is entrapped within sediment but not in de
-floc easy to redisperse but de, difficult to redisperse
What is the difference between flocculation and coagulation?
flocs are temporary as they have a loose structure where the particles are a small distance apart and are weakly bounded to each other. coagulation arises from particles that are closely aggregated and hard to redisperse. It’s permanent and leads to caking.
Why is caking suspensions bad?
leads to pt underdosing or overdosing
What can we do to reduce caking? (3)
-add viscosity-enhancing agents, flocculating agents, wetting agents/surfactants
What can we do if viscosity is too high?
Add flocculating agent
Why is too flocculated system bad?
because the aggregates will sink too quickly. So needs to be partially deflocculated system for best suspension
Give 3 examples of flocculating agents (which you must know)
-electrolytes i.e. salts
-surfactants e.g. wetting agents
-polymers
these prevent coagulation and therefore caking (and so do wetting agents)
What is the occurrence of coagulation and/or flocculation driven by?
electrical forces
All particles tend to have a…
surface charge
Do all the drug particles in a suspension have the same charge/surface charge? Why or why not?
yes
because they are made up of the same drug
Do the particles in a suspension repel each other? Why or why not?
yes. like charges repel. they have the same surface charge (++ repel . - -) + and - will not repel
Are there only repulsive forces between the drug particles in a suspension?

What are VDW forces?
anything that isn’t hydrogen or ion-ion
What theory does flocculation depend on?
DLVO theory
What is the equation for DLVO theory?
VT = total potential energy of interaction;
VA = potential energy of attraction;
VR = potential energy of repulsion
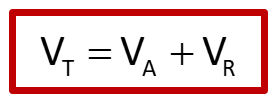
What does VR determine?
whether a partially deflocculated system is stable or not
What does VR depend on? (2) What is VR?
-surface charge
-the thickness of the double layer
both govern zeta potential
repulsive forces
What does the surface charge of the particles cause in a suspension?
-causes the particles to interact with each other and with the liquid medium in which they are dispersed in.
What do the ions in the vehicle of a suspension repel?
they repel the particle surface if they are the same charge as it
Explain the electrical property in colloids
The counter-ions in the suspension are attracted to the drug particles’ surface, therefore will form strong interactions with it. They will also ‘cancel out’ some of the surface charge. The extent depends on the number of counter ions in the solution. The higher the net charge, the greater the strength of the repulsive forces. Therefore, particles with high net charges will repel each other very strongly and will be kept far apart, which leads to good dispersion and no flocs. BUT, the particles will sediment very slowly as they aren’t in flocs, so there will be caking as a result.
Attractive forces e.g. +-, VDW and LDF and delta + and delta - will always outweigh repulsive forces
-if you want to encourage floc formation, one way is by controlling net surface charge and repulsive forces. Zp is a good proxy for figuring out surface charge.
Why is it bad to add too many electrolytes in a suspension?
repulsive forces can increase again because zeta potential becomes negative. You don’t want zeta potential being too high or low
-you want there to be some repulsion/weakening of attraction between drug particles to avoid coagulation but also not high to the point where there are 0 flocs formed.
What does the value of VT depend on?
how far apart the particles are
What does a zeta potential close to zero mean?
little repulsion of particles
Explain, in electric terms, what is happening when there is a particle with a negative surface charge. Explain the DLVO theory
-particles with a like surface charge will repel other particles of the same surface charge. The higher the net surface charge, the higher the repulsive forces. This allows good dispersion because the particles are further apart. But, they won’t form flocs, so there will be caking (densely packed sediment) since there’s no liquid trapped.
-The negative charge at the particle surface will attract positive ions (counterions) in solution, forming strong electrostatic interactions. This will ‘‘cancel out’’ some of the negative charge (make it less negative), which decreases zeta potential. Because of more positive ions present, there will also be some negative ions. The more counterions present on its surface, the lower the zp.(Don’t want it too low otherwise it will be negative and be repulsion)
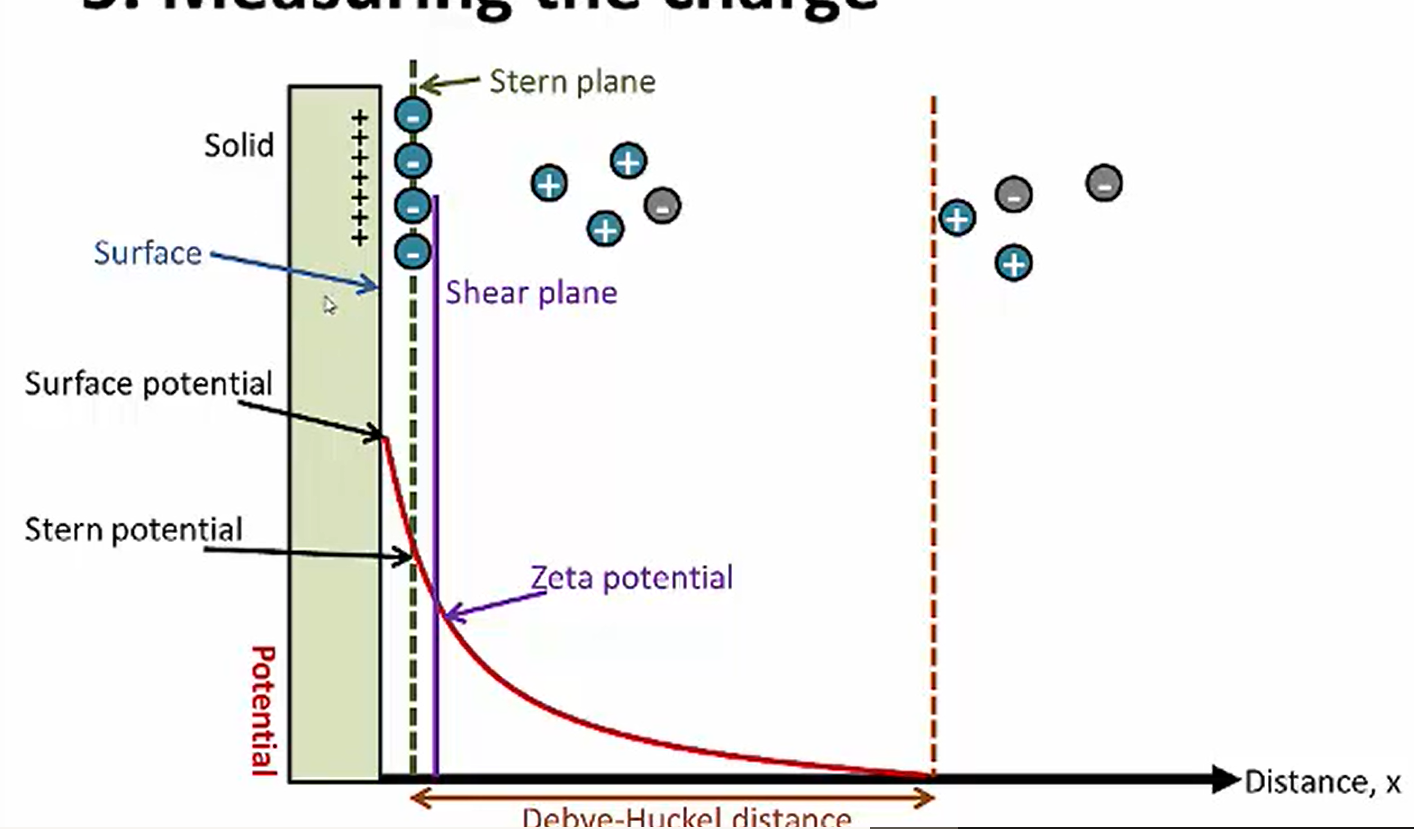
-the electrical double layer consists of the diffuse layer and stern plane. This layer is as thick as the size of a hydrated counter-ion (an ion that’s surrounded by water molecules).
-stern plane= the counterions that are permanently stuck to the particle surface
-diffuse layer= the mixture of + and - ions in the vehicle. These ions bind weakly to the drug particle surface
-The "shear plane" is an imaginary boundary where the liquid particles adhering to the particle’s surface behaves differently from the liquid particles farther away in the continuous phase (the bulk liquid) . The liquid farther away from the particle is free to flow without sticking to the surface.
-The closer you are to the particle, the more positive ions (counter-ions) are present. As you move farther away, there’s more negative ions (co-ions). Eventually, the Debye-Hückel distance is reached, which is the distance between the stern plane and where the the number of positive ions and negative ions are about the same in the suspension (charge/electrical potential=0).These ions don’t interact with the particle surface
-attractive forces result from VDW forces
How would the DLVO theory look on a graph? Label this graph
"Cake First, Floc Later"
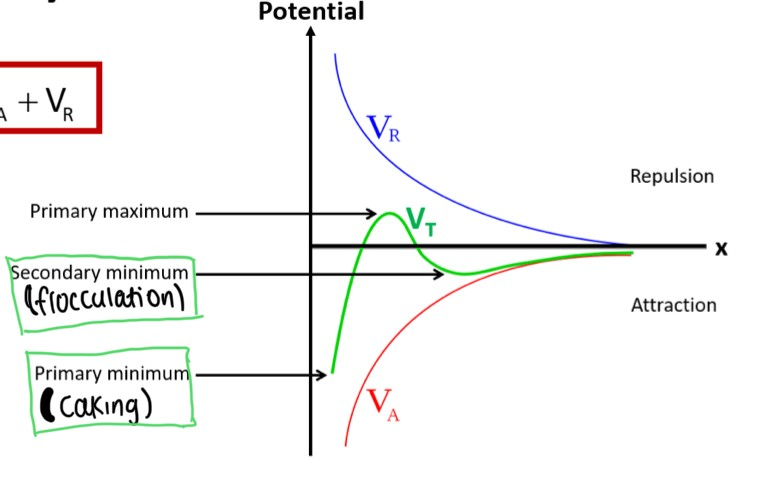
What does VR affect on the DLVO graph?
the height of the primary maximum and the depth of the secondary minimum energies (2 closest things to it)
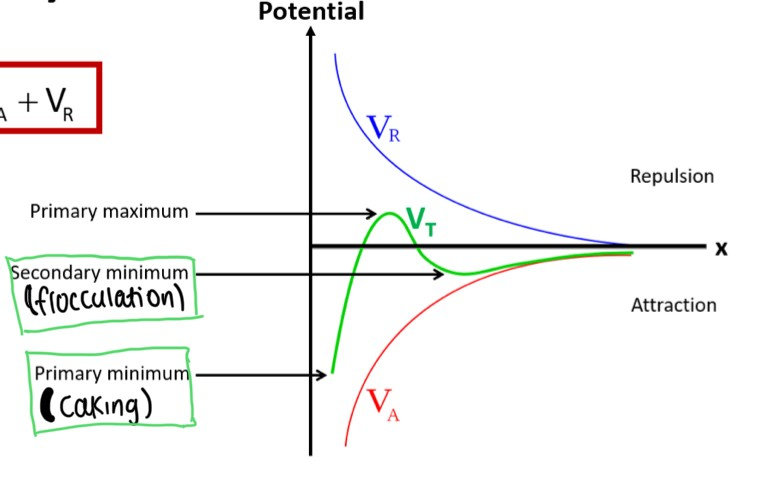
What is meant by an ‘energy minimum’ on a DLVO graph?
-when the attractive forces between close-together particles in the colloid dominate (leading to caking)
What is meant by an ‘energy maximum’ on a DLVO graph?
-At intermediate distances, the double layers repel. If this maximum is large, the particles will stay dispersed. If it is small, the particles will aggregate
Relationship between electrostatic double layer and repulsion
thicker double layer=more repulsion=more even dispersion
What ALWAYS overpowers, attractive or repulsive forces?
attractive
Electrolytes change the…
-net surface charge of a particle
Does VA ever change?
-never
Explain what happens if i add electrolytes into a colloid/suspension in terms of DLVO theory
-VR changes because the depth of the secondary minimum increases when the particles adsorb onto the counter ions. This allows for floc formation because electrolyte addition decreases repulsion, so zeta potential decreases. Decreased repulsion also means decreased thickness of the electrical double layer. Debye Huckel distance decreases (How "thick" the electric double layer is — or how far a particle can “push away” others with its charge)
-do not add too much as it will lead to caking due to zeta potential becoming too low
-electric double layer, which creates electrostatic repulsion between particles — helping keep them dispersed.
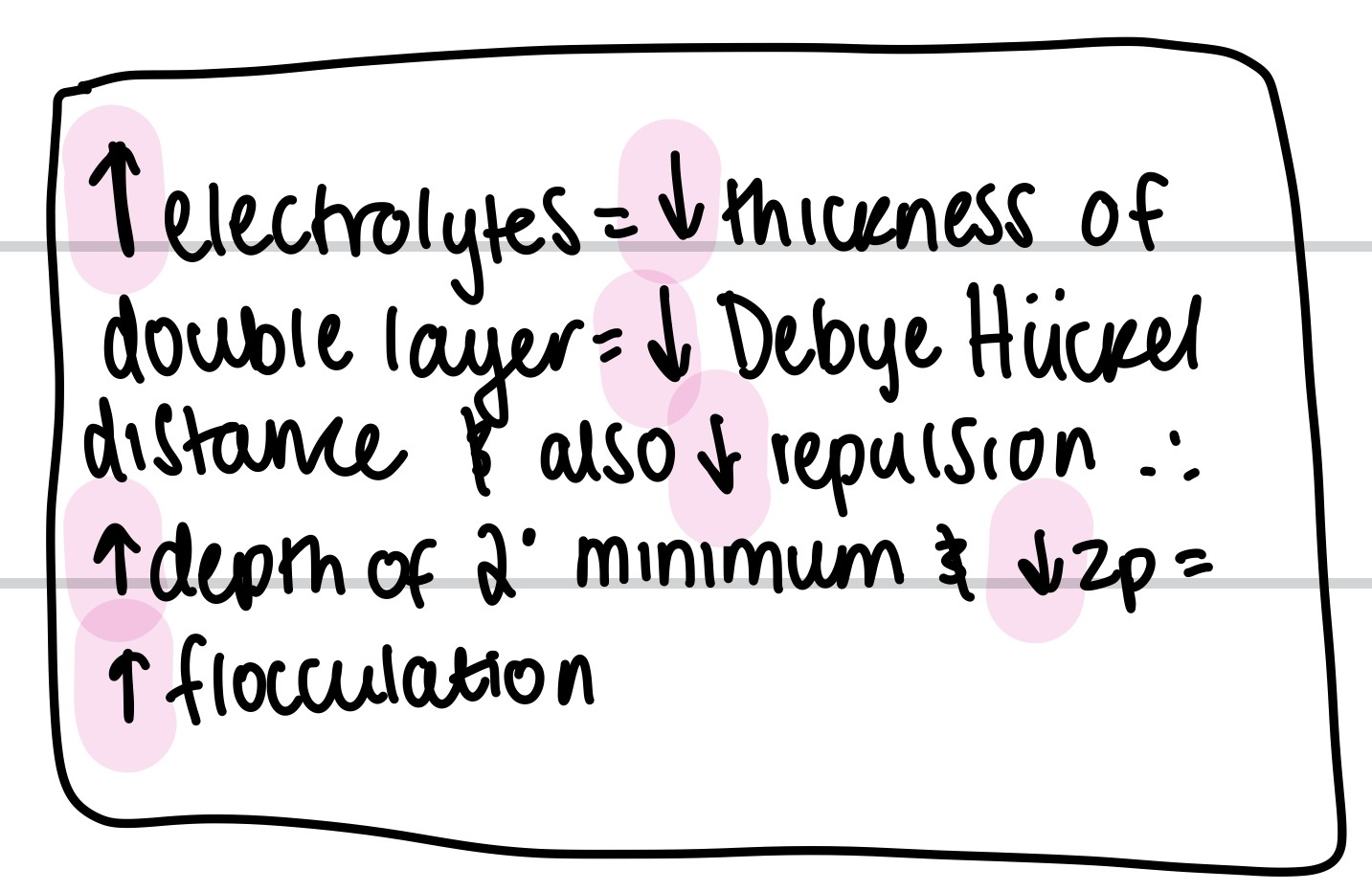
What 3 things can be added to reduce caking? (4)
flocculating agents= polymers, electrolytes, surfactants
wetting agents
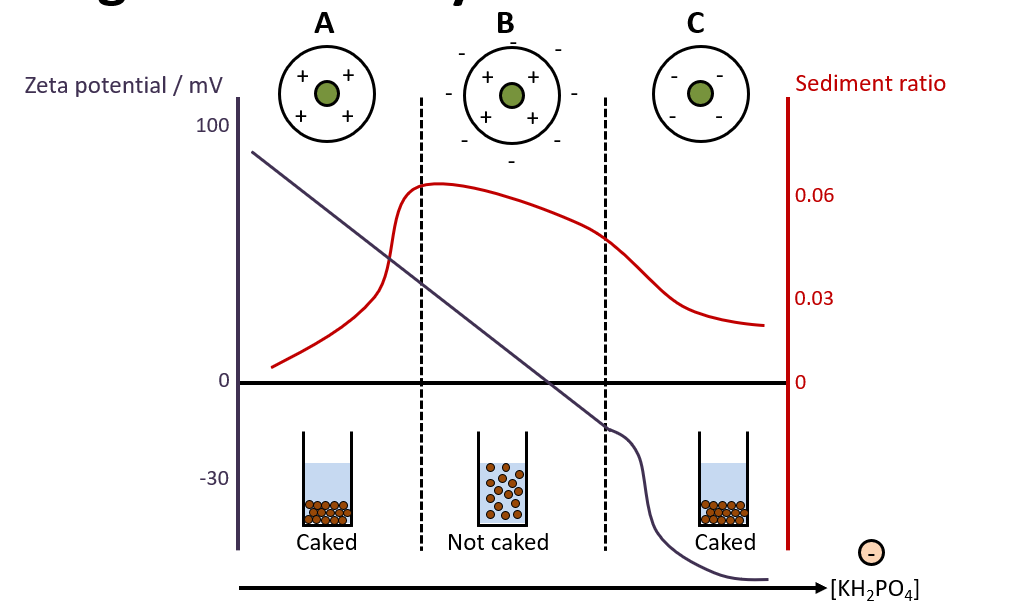
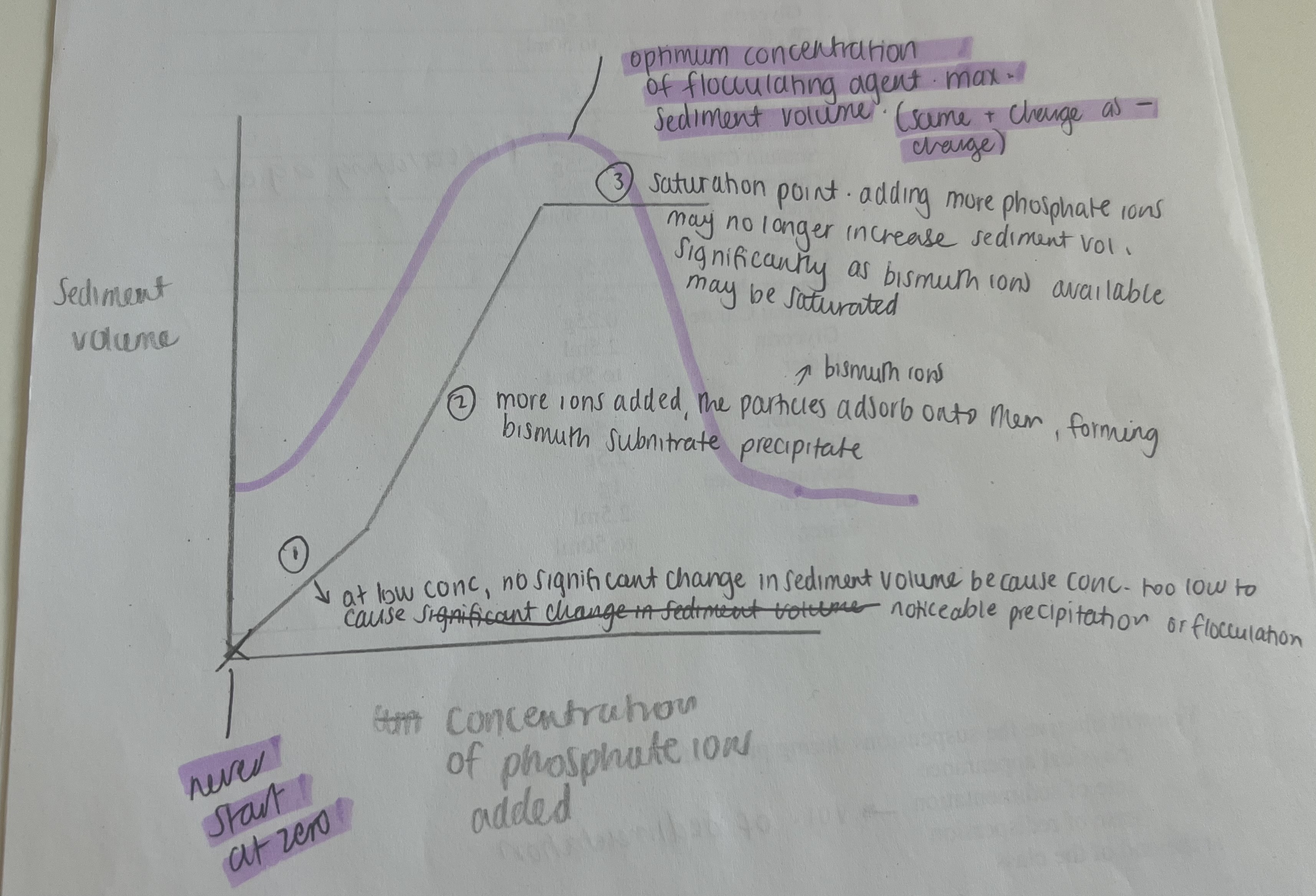
Explain this graph (to do with bismuth subnitrate particles and adding KH2PO4)
Bismuth subnitrate particles have a + charged surface. Initially, the system is deflocculated, so there is caking. At high positive (Region A) or high negative (Region C) zeta potential, particles repel each other and remain dispersed. Over time, these repelled particles settle individually, forming a densely packed sediment. This results in a hard, compacted cake, which is difficult to redisperse. Adding KH2PO4/electrolyte causes a reduction in the zeta potential of the particles when the PARTICLES ADSORB ONTO THE PHOSPHATE IONS i.e. the counter ions. As more KH2PO4 is added, the zeta potential reduces to zero and then turns negative (when too much KH2PO4 is added). To obtain a partially deflocculated anti-caked system, you need to control the zeta potential by adding correct amount of electrolyte.
-Ideally, zeta potential should be low/high enough to allow settling but not so low that particles form a hard cake. (not too high, not too low)
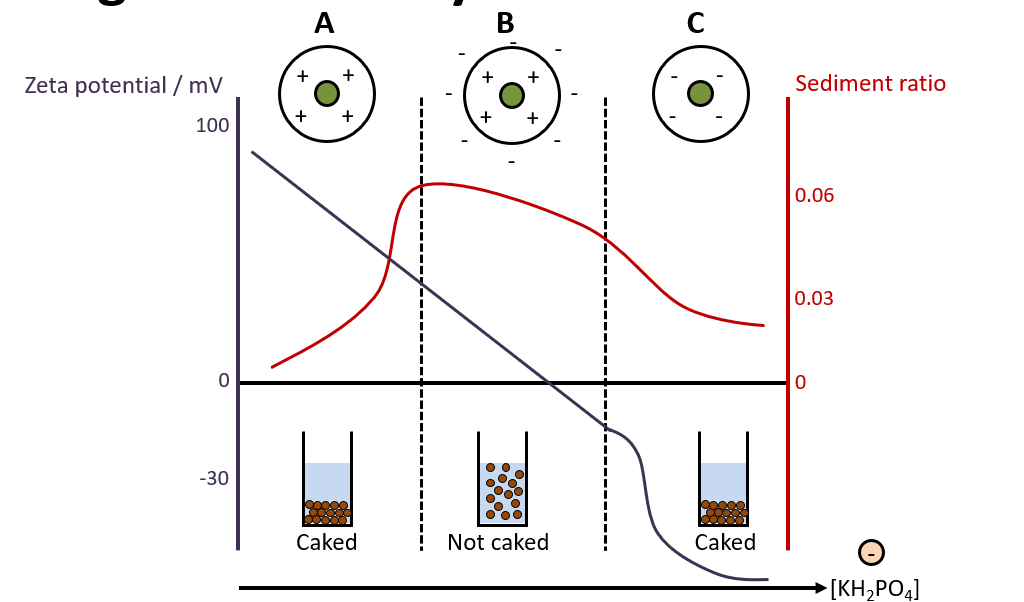
Why does adding surfactant reduce caking?
-adding anionic or cationic surfactants can neutralise the surface charge of a particle, hence reduce repulsive forces between them, helping with flocculation. There should be some attractive and some repulsive forces for a good suspension!!!!
-in this case, same explanation as adding electrolytes
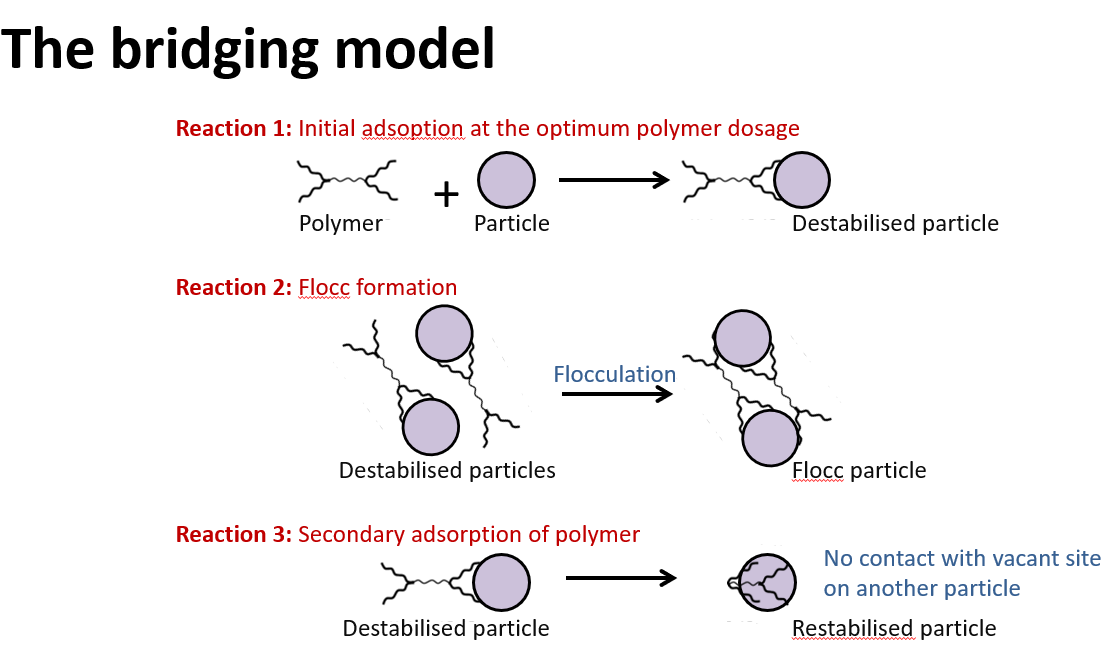
Why can adding polymers into colloid/suspension reduce caking?
-the functional groups of the polymers interact with the surfaces of the particles.
-polymer adsorbs onto drug particle. the free-end of the polymer adsorbs onto another particle. This gives interparticle bridging, leading to flocculation. BUT, if there are no other particles to interact with, the free end of the polymer coats the particle its adsorbed to instead. This leads to restabilisation and a deflocculated system (not good) so need to make sure right amount of polymer is added or not too much.
Illustrate below with a sketched graph how you expect the sediment volume to change as increasing amounts of phosphate ions are added to bismuth subnitrate suspension
adding too much causes coagulation, therefore still sediment volume but decreases as there is no liquid entrapment
Give 2 formulation measures you could take to reduce the sedimentation rate of solid drug particles in a suspension
smaller particle size e.g. turning it into powder
-increase viscosity by adding viscosity-enhancing agent
-flocs don’t reduce the rate
Glycerin is a wetting agent. What does this mean it does?
it wets hydrophobic drug particles
Give an example of an electrolyte
sodium citrate
KH2PO4
Give examples of polymers
silicates
cellulose derivatives

-2.56×10^-4 mg/s
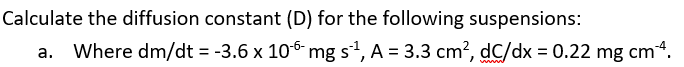
4.68×10^-6 or 4.96×10^-6

Your assistant is clearly wrong – a more concentrated suspension will have a higher turbidity value, not a lower one.
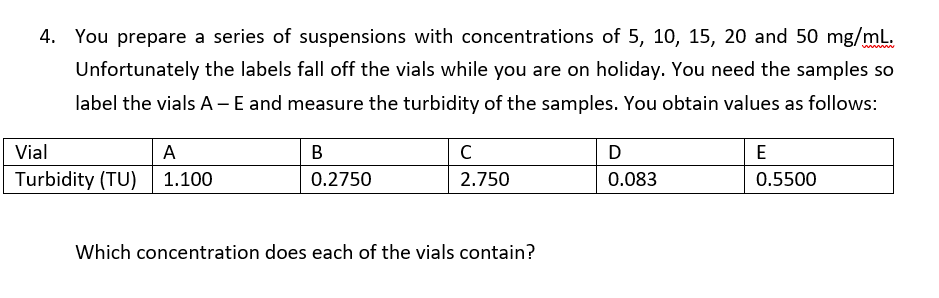
C=highest concentration
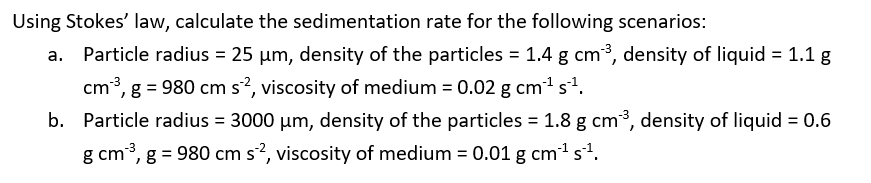
Remember to convert um3 to cm3 so that the units are consistent
1 um3 = 10^-12 cm3
1.8×10^-17cm s-1
2.1×10^-12cm s-1
Can caking be prevented?
NO DO NOT SAY PREVENT SAY REDUCE OR SLOW
Why do we want to avoid caking?
To maintain dose uniformity
What type of forces do repulsive forces arise from?
Electrostatic forces
What type of forces do attractive forces arise from?
VDW forces
If drug is mainly hydrophobic and it’s clumping and clinging on the walls, what should be added?
Wetting agent
What do viscosity-enhancing agents enhance?
The viscosity of the continuous phase, so that the particles settle slower so it doesn’t cake so quick
What type of motion do colloids undergo in a suspension?
They undergo Brownian motion, which is rapid and random
What is Fick’s first law? (definition)
-movement of particles (diffusion flux) from high to low concentration is directly proportional to the particle’s concentration gradient