atomic orbitals and electron filling
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
what happens to energy levels as n increases
get closer together and converge at n = ∞ where there is zero energy. more negative energy = more stable.
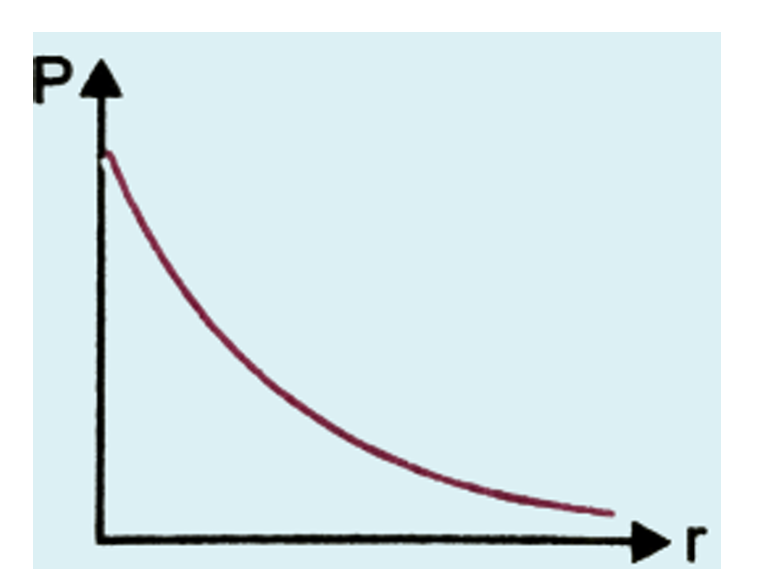
what is a probability density plot
what are the problems with it?
exponential curve showing probability density against distance from the nucleus
problems: never hits zero, probability highest at r = 0 ie at the nucleus (but there are no electrons found here).
what was developed to avoid the problems with a probability density plot
a radial probability plot, which shows the probability against distance as well, but absolute probability is not plotted - instead it is normalised (see 1205)
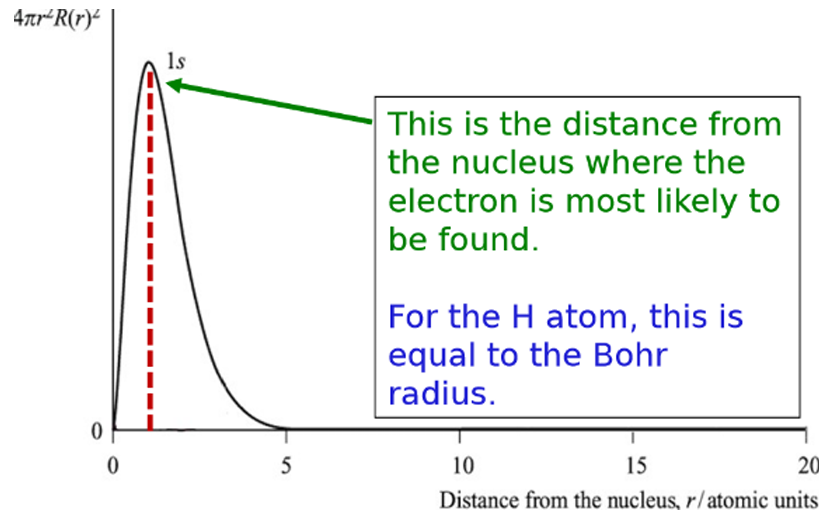
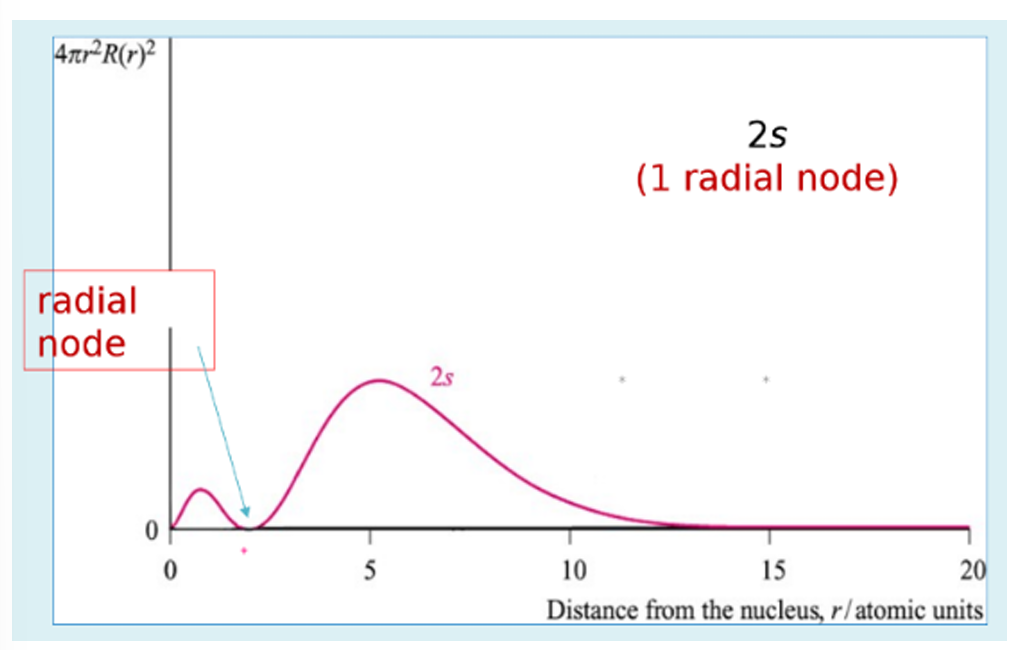
radial probability plot for 2s orbitals
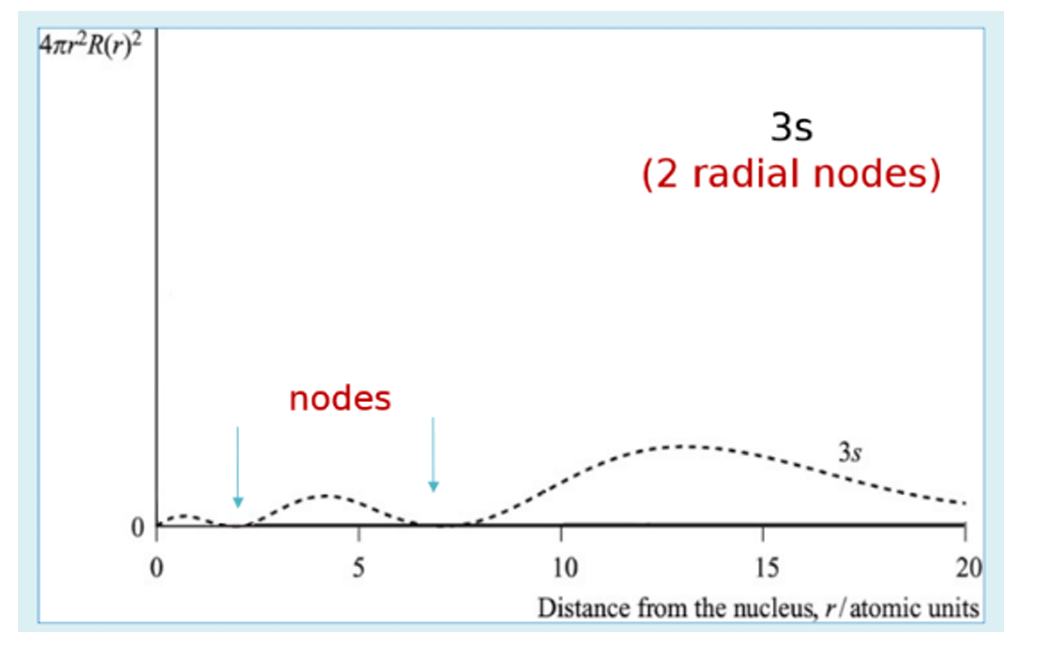
radial probability plot for 3s orbitals
what is orbital penetration and why does it occur
penetration power of the orbitals?
p-orbitals have a zero-point at the nucleus but s-orbitals don’t.
electron penetration is the electron’s probability density near the nucleus, and so as s orbitals have a higher density near the nucleus, they are penetrating the nucleus of the atom more than the p orbitals. this results in them having lower energy.
penetration power: s > p > d > f
name the 3 rules for electron distribution within orbitals
Aufbau Principle
Pauli’s Exclusion Principle
Hund’s Rule of Maximum Multiplicity
what is the aufbau principle
orbitals are filled with electrons in order of increasing energy
what is pauli’s exclusion principle
no two electrons in the same atom can have the same set of four quantum numbers, so if two electrons occupy the same atomic orbital, they must have different spin quantum numbers. these two electrons are said to be spin-paired.
what is hund’s rule of maximum multiplicity
electrons occupy degenerate orbitals singly before pairing up