Local Anesthetics
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
definition of local anesthetic
local anesthesia refers to loss of sensation in specific region of the body
Delivered directly to the target organ/tissue
Recovery is spontaneous, predictable, with no residual effects

local anesthetics MOA
Reversible inhibition of action potential conduction in sensory neurons
Anesthetic mechanism of action: block voltage-gated sodium channels
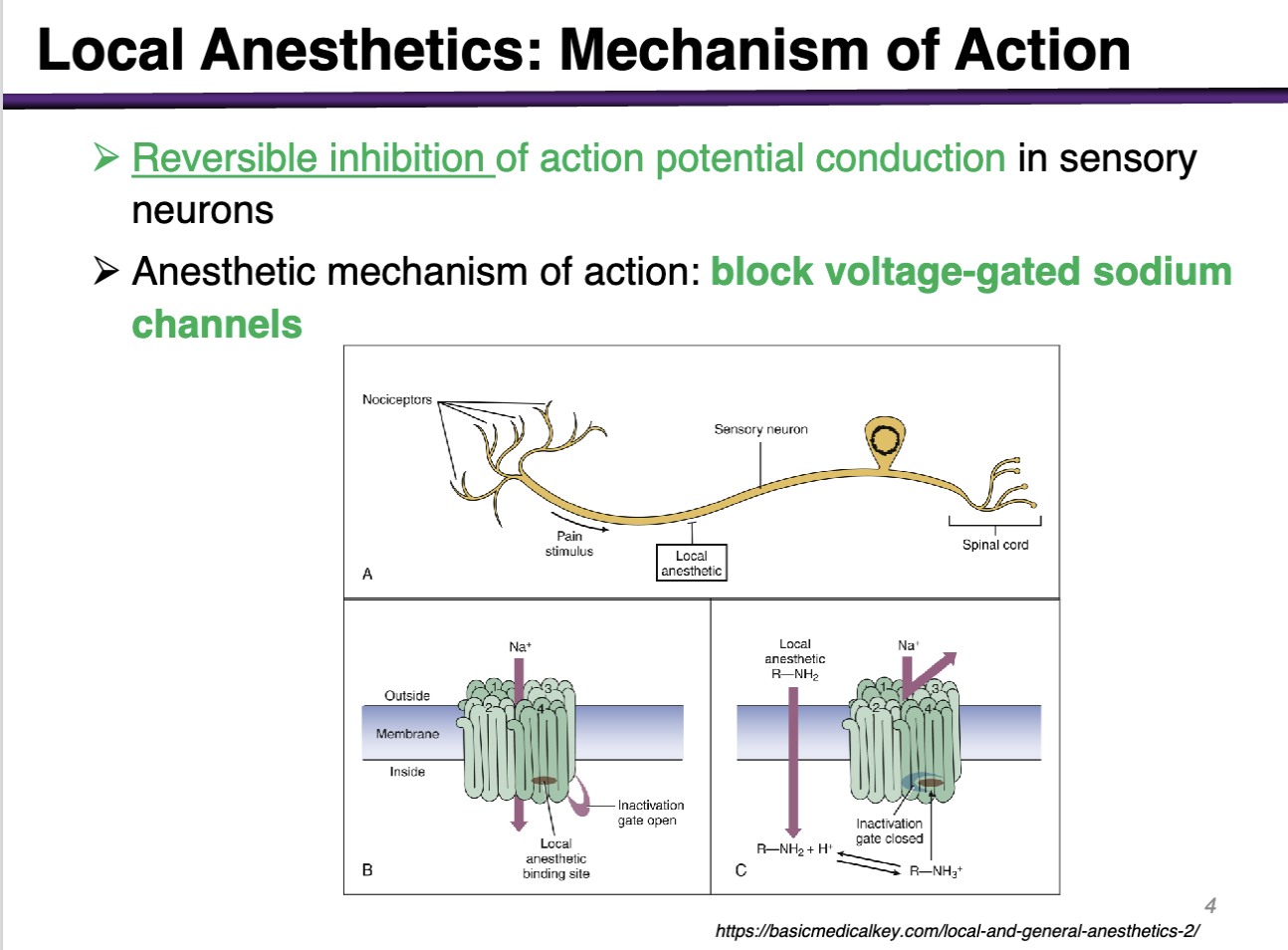
Where does the conduction of action potential occur in the neuron?
Blocking voltage gated sodium channels Along the axon
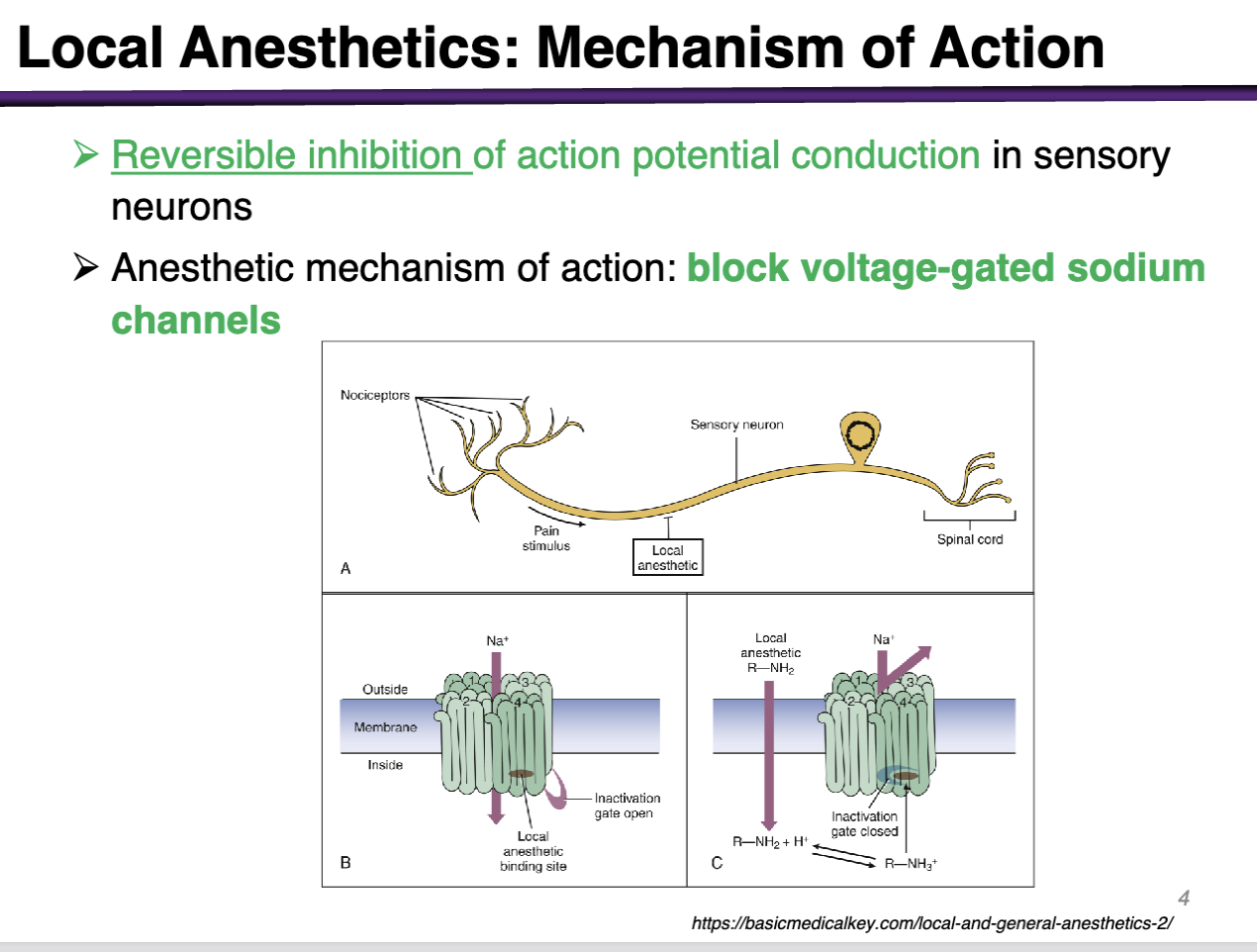
Review of action potential
1. Resting potential of -70 mV
2. Excitation (depolarization) reaches threshold; leads to sodium channel opening
3. Continued depolarization causes sodium channels inactivation as potassium channels begin to open
4. K+ and Na+ re-establish equilibrium conditions, channels return to resting state
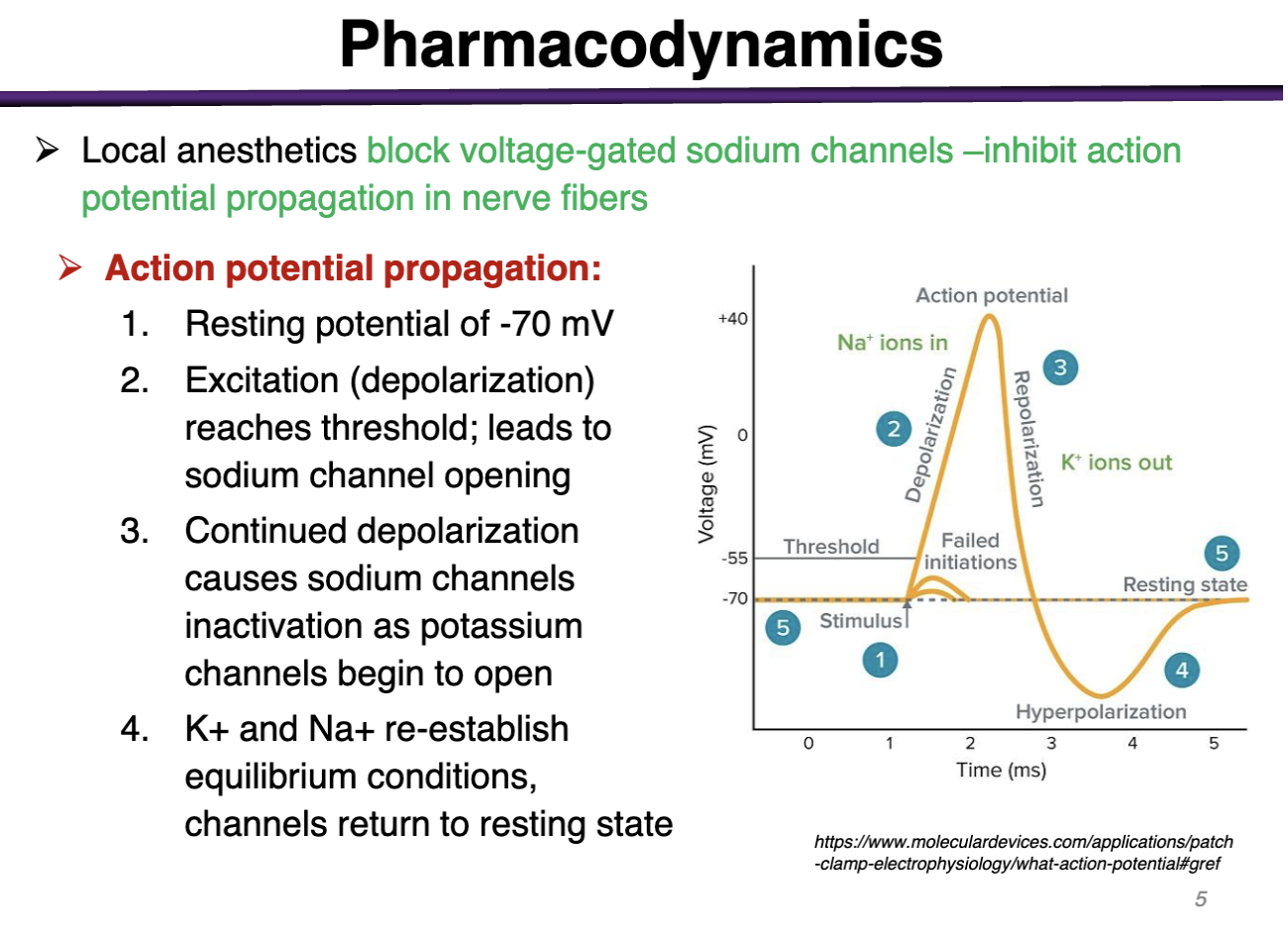
Sodium Na channels exist in what states
Resting, open, inactive
Sodium has the inactivation gate
Potassium only has 2 states: Open or Closed
Local Anesthetic Molecule
Amine is hydrophilic (ex is it charged or uncharged?)
local anesthetics are the most active in the charged state
Local anesthetics are classified via ester or amide linkage
Lipophilic part is aromatic ring
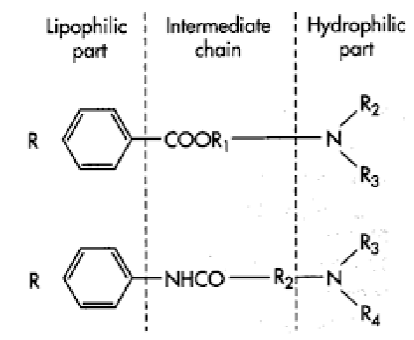
what is the lipophilic part of the local anesthetic
Aromatic ring is lipophilic: required to cross the cell membrane
The charged form is most active at the receptor (channel binding) but the receptor site is intracytoplasmic
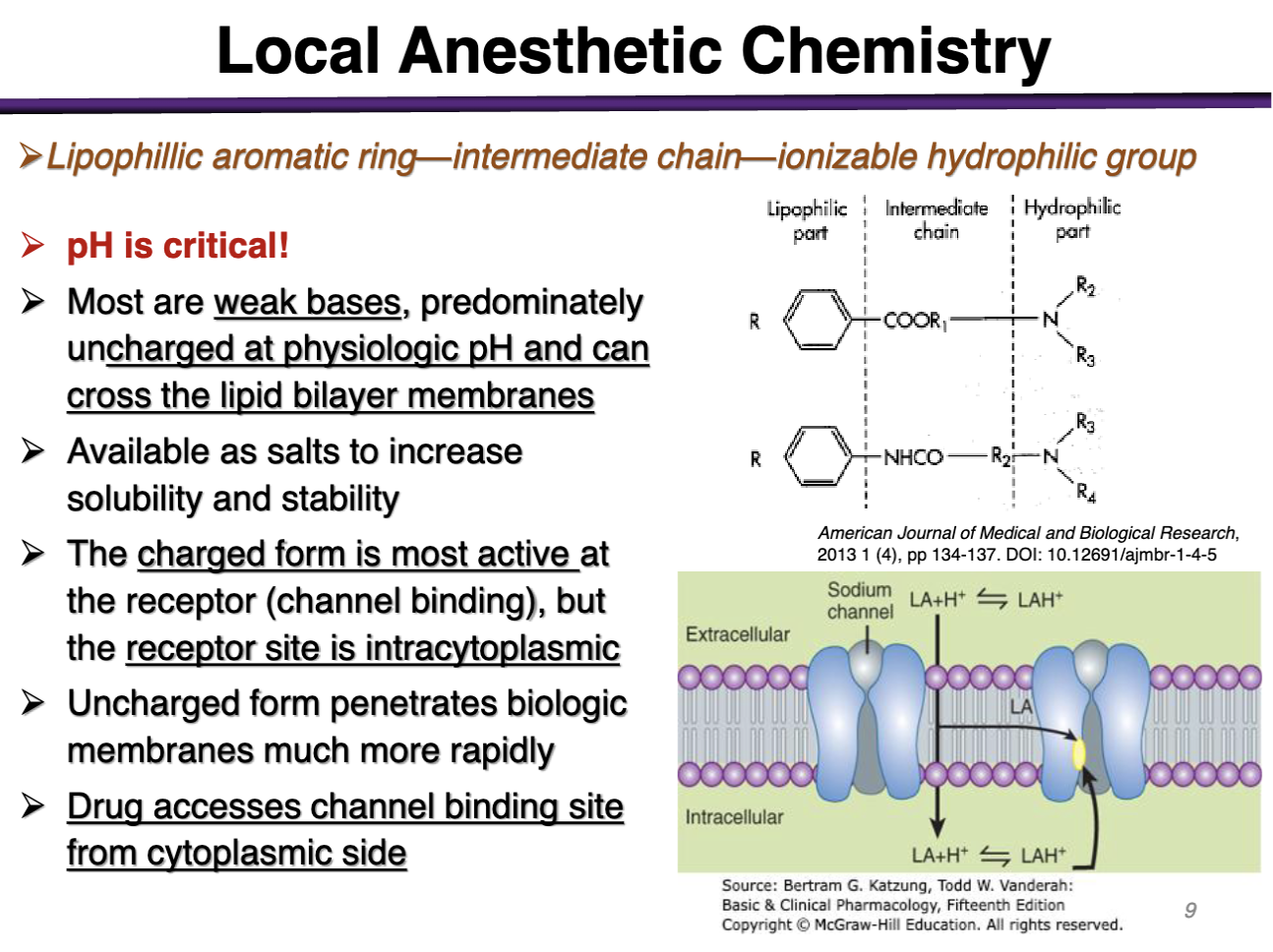
T/F local anesthetics need to be in the uncharged form to bind to the voltage gated Na channel
FALSE. They have to be charged!
The charged form is most active at the _______ but the receptor site is ______
The charged form is most active at the receptor (channel binding) but the receptor site is intracytoplasmic
Must be uncharged at physiologic pH to cross the barrier and then they become charged intracellularly to become charged and bind inside the cytoplasm
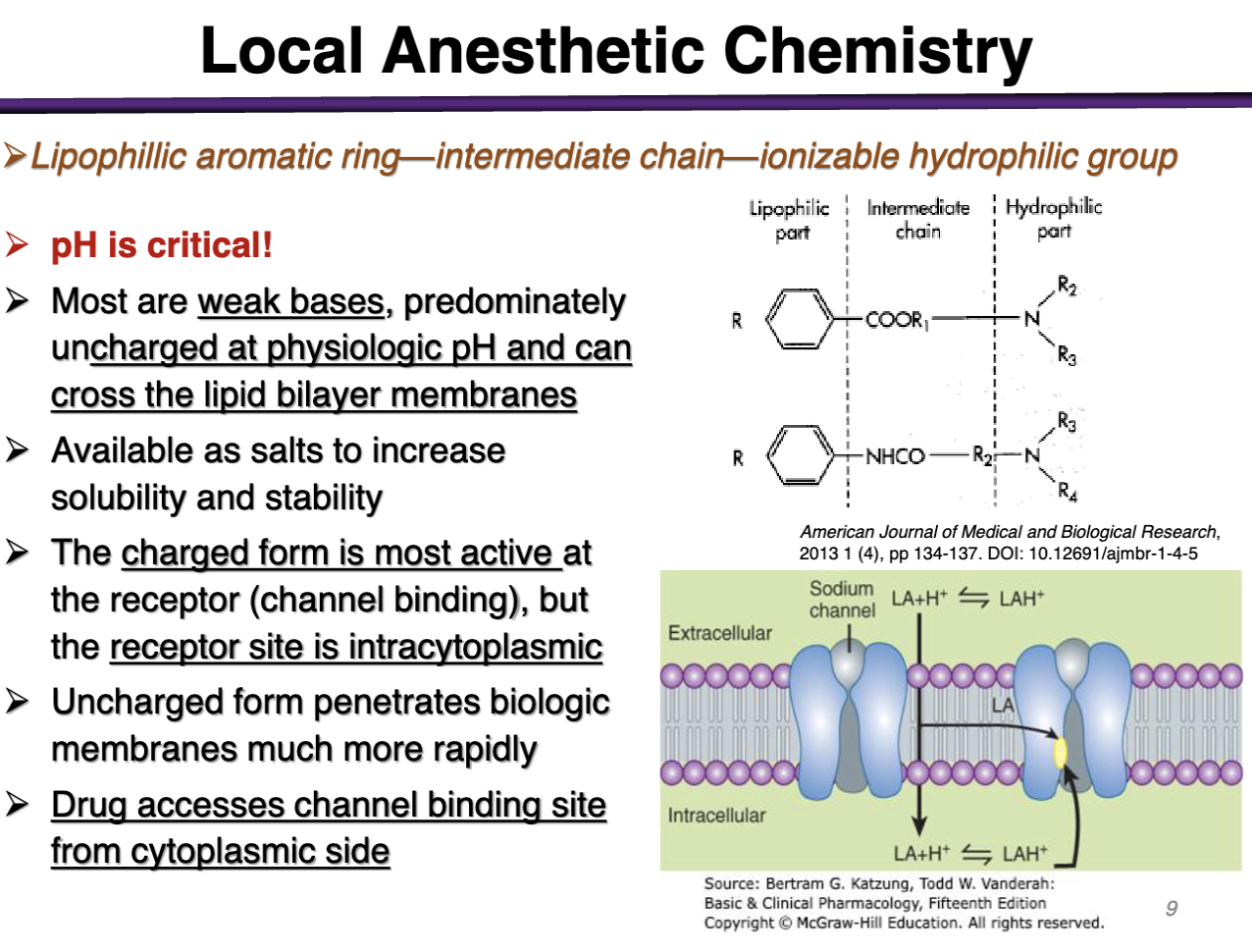
anesthetic drugs are given with CO2; why?
CO2 can cross the cell membrane; resulting an in acidifying environment (after crossing); the local anesthetic can now become protonated after crossing to become more charged
(CO2 increases intracellular space, cells accumulate the charged form of the drug)
-repeated use would acidify the extracellular space
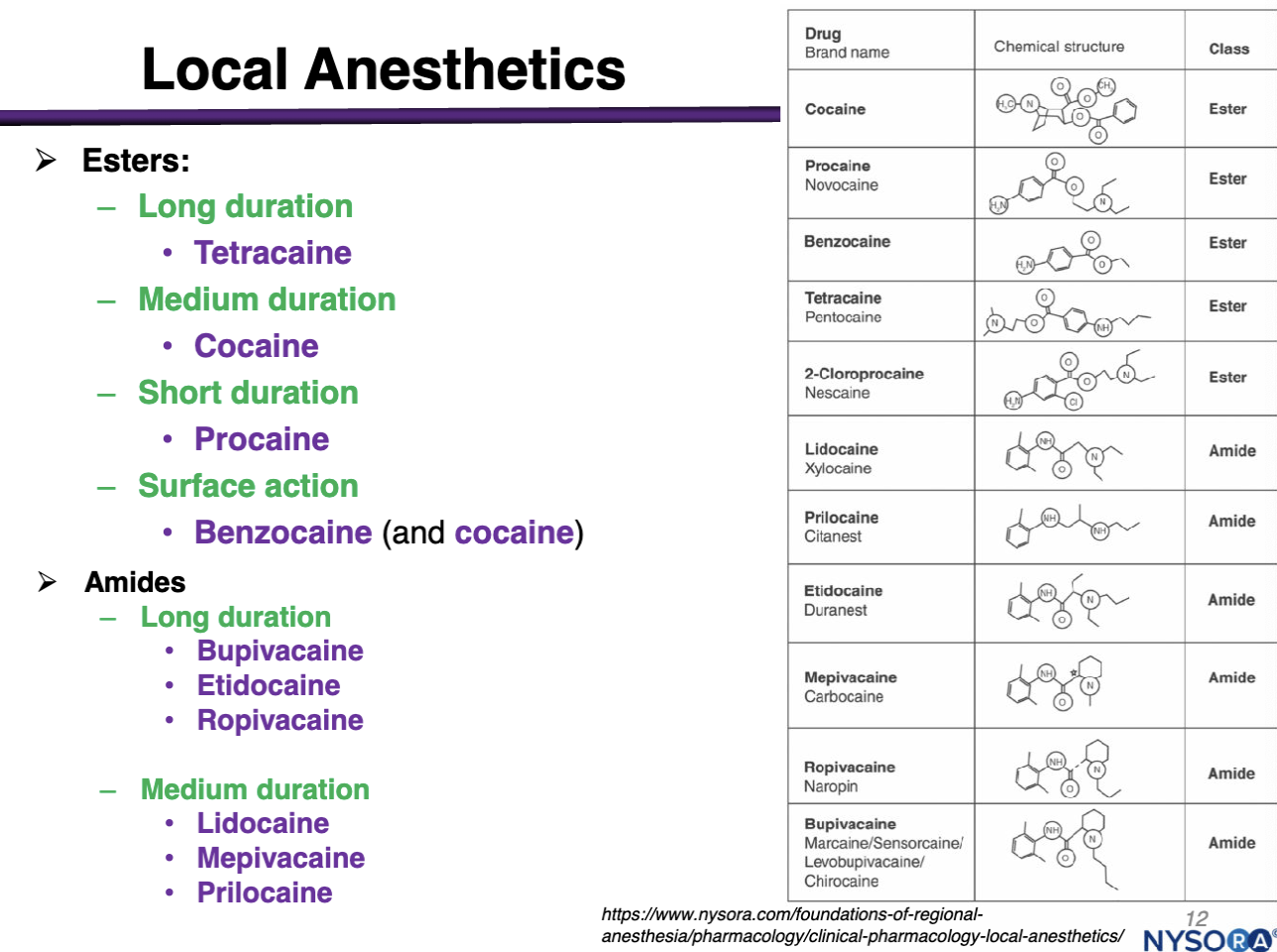
Examples of amides
Long Duration:
Bupivacaine
Etidocaine
Ropivacaine
Medium Duration:
Lidocaine
Mepivacaine
Prilocaine
Long duration amides
Bupivacaine
Etidocaine
Ropivacaine
Medium duration amides
Medium Duration:
Lidocaine
Mepivacaine
Prilocaine
Examples of Esters
Long Duration: Tetracaine
Medium Duration: Cocaine
Short Duration: Procaine
Surface Action: Benzocaine and cocaine
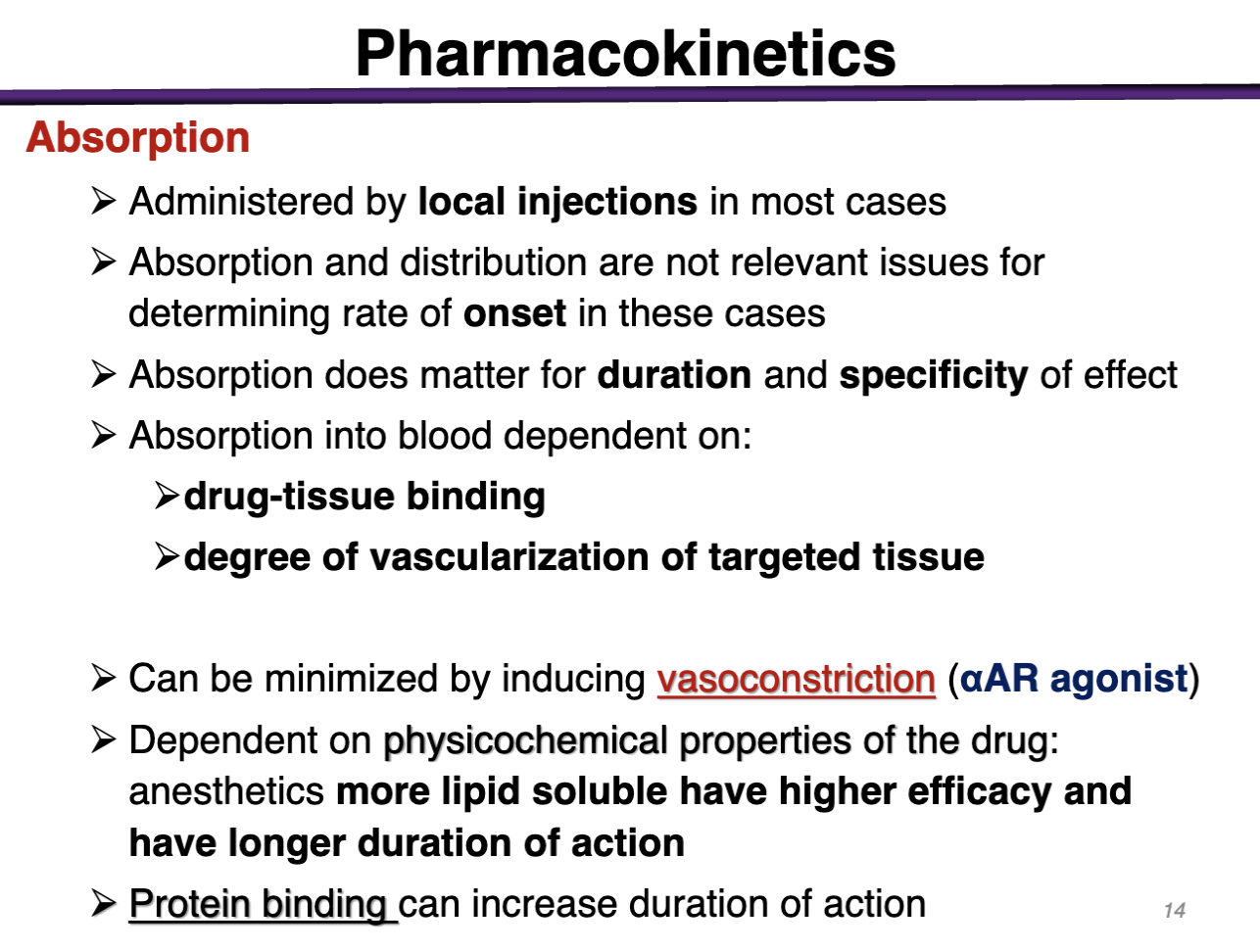
Absorption of local anesthetics
Administered via local injections
Absorption and distribution is not relevant for determining rate of onset (THESE ARE NOT SUPPOSED TO ENTER SYSTEMIC CIRCULATION)
Absorption into blood is dependent on:
Drug-tissue binding
Degree of vascularization of targeted tissue
what is absorption of local anesthetics dependent on?
Absorption into the blood is dependent on:
Drug-tissue binding
Degree of vascularization of target tissue
Dependent on physicochemical properties of the drug: anesthetics more lipid soluble have higher efficacy and longer duration of action
Protein binding can also increase duration of action because proteins do not get eliminated in the kidney
Distribution is not relevant because for onset because we WOULD NOT WANT THIS IS SYSTEMIC CIRCULATION
how can you prevent systemic absorption of a local anesthetic
Can be minimized by inducing vasoconstriction (aAR agonist)
could give epinephrine to vasoconstrict to reduce risk of systemic circulation
Decreases blood flow and distribution of anesthetic away from injection site
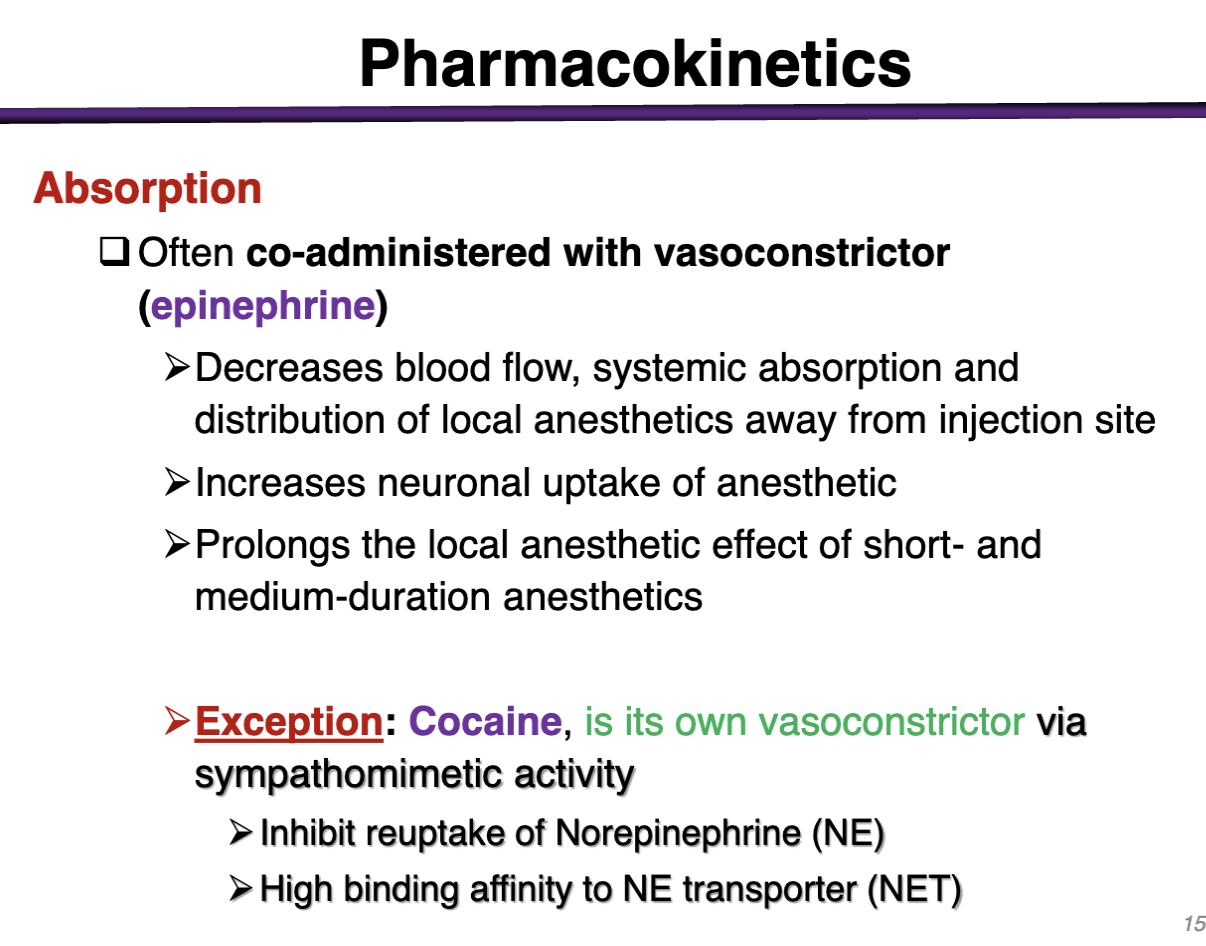
What local anesthetic does not need to be coadministered with epinephrine?
COCAINE
is its own vasoconstrictor via sympathomimetic activity
Inhibit reuptake of NE and high binding affinity to NE transporter NET
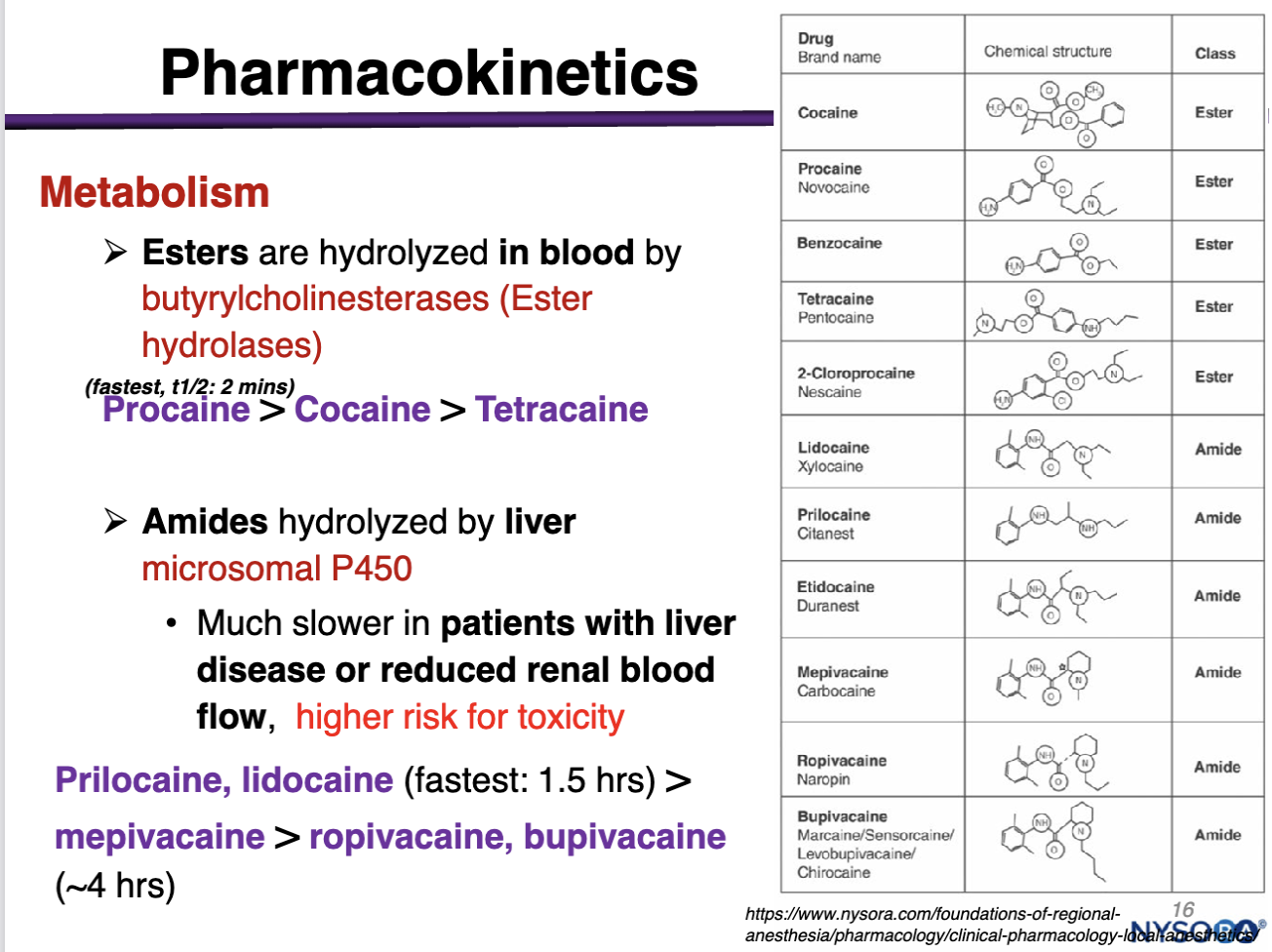
Esters are hydrolyzed in the blood by
ester hydrolases made in the liver and found in systemic circulation
procaine>cocaine>tetracaine
(within minutes)
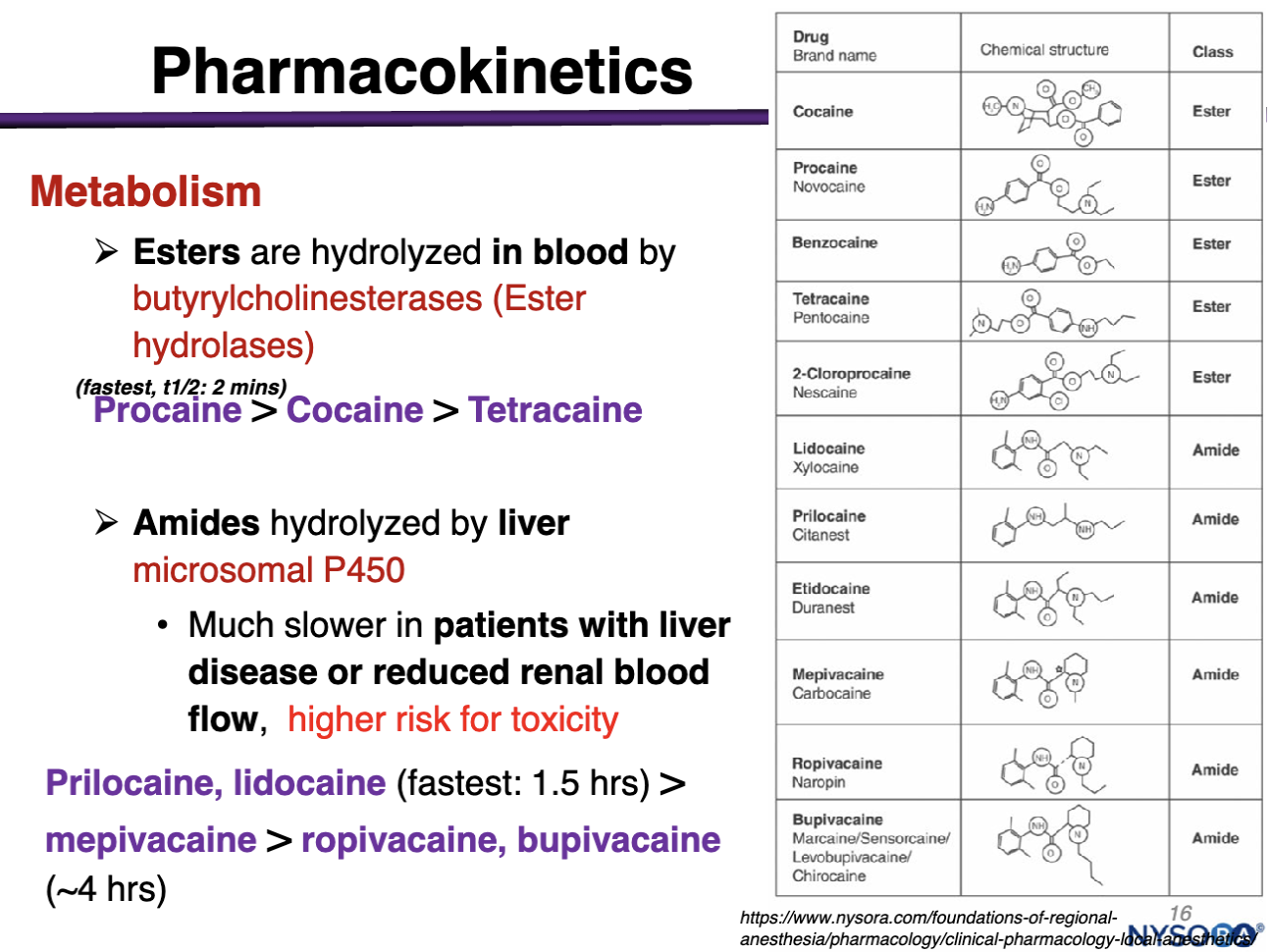
amides are hydrolyzed by
liver microsomal P450; NOT Blood
Degradation/Elimination is Much slower in pts with liver disease or reduced renal blood flow, high risk of toxicity
Prilocaine, lidocaine (1.5 hours) > mepivacaine > ropivacaine, bupivacaine (4 hrs)
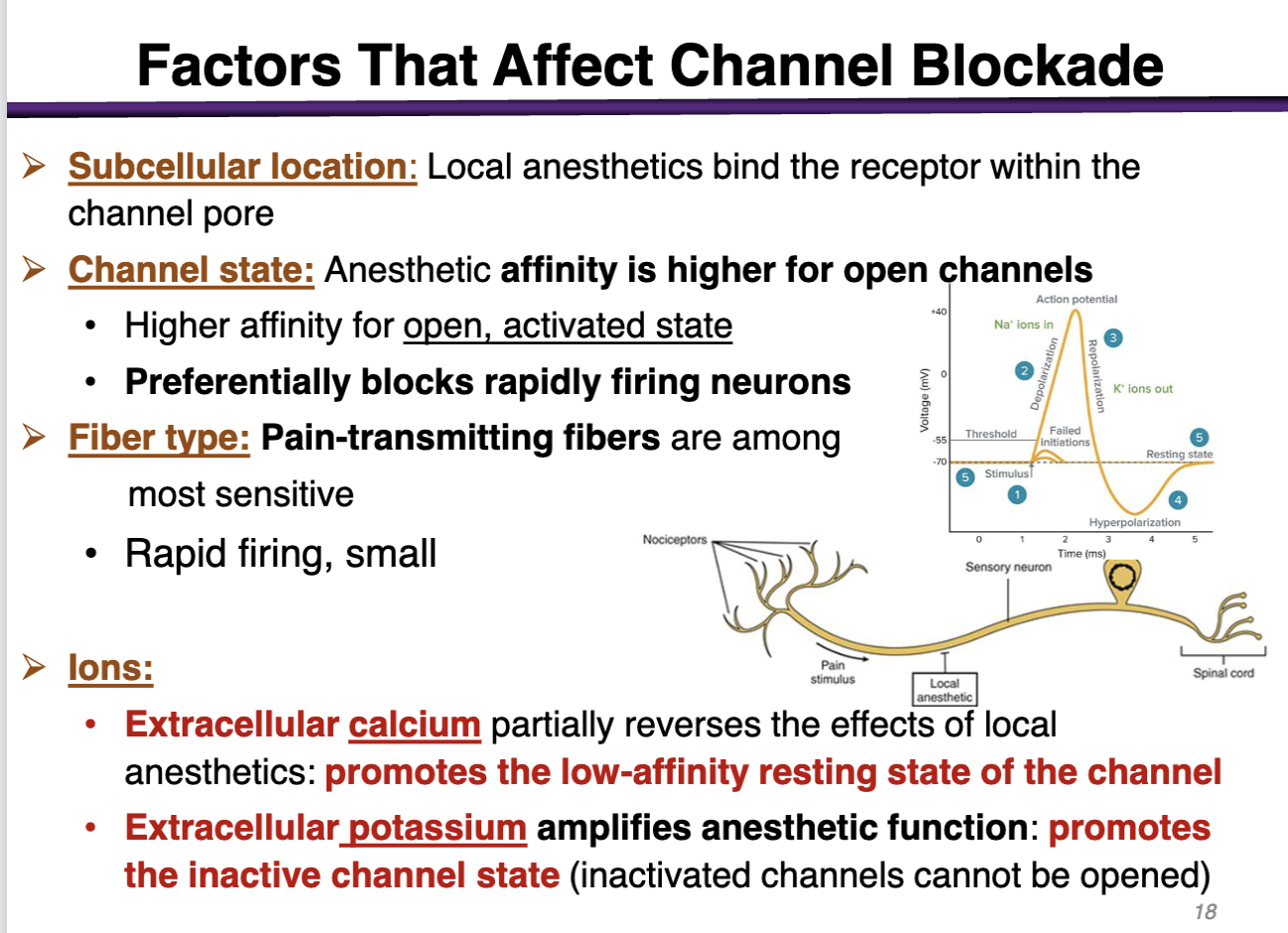
how can subcellular location factor affect channel blockade
The target is located subcellular (inside the cell membrane); the drug has to cross the cell membrane and go to the cytoplasm
local anesthetics bind the receptor within the channel pore
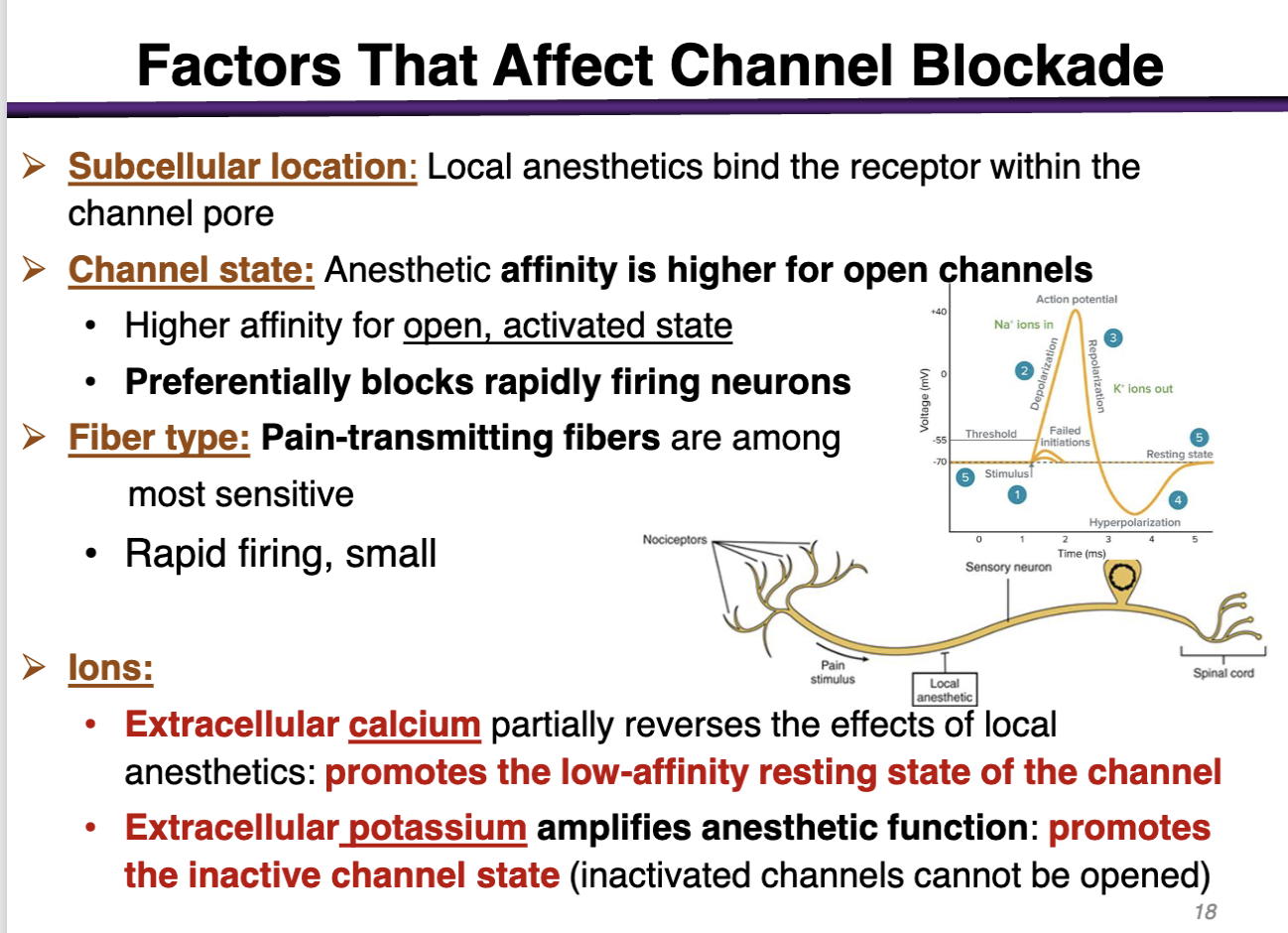
how can channel state factor affect channel blockade
anesthetic affinity is higher for open channels
preferentially blocks rapidly firing neurons (during depolarization)
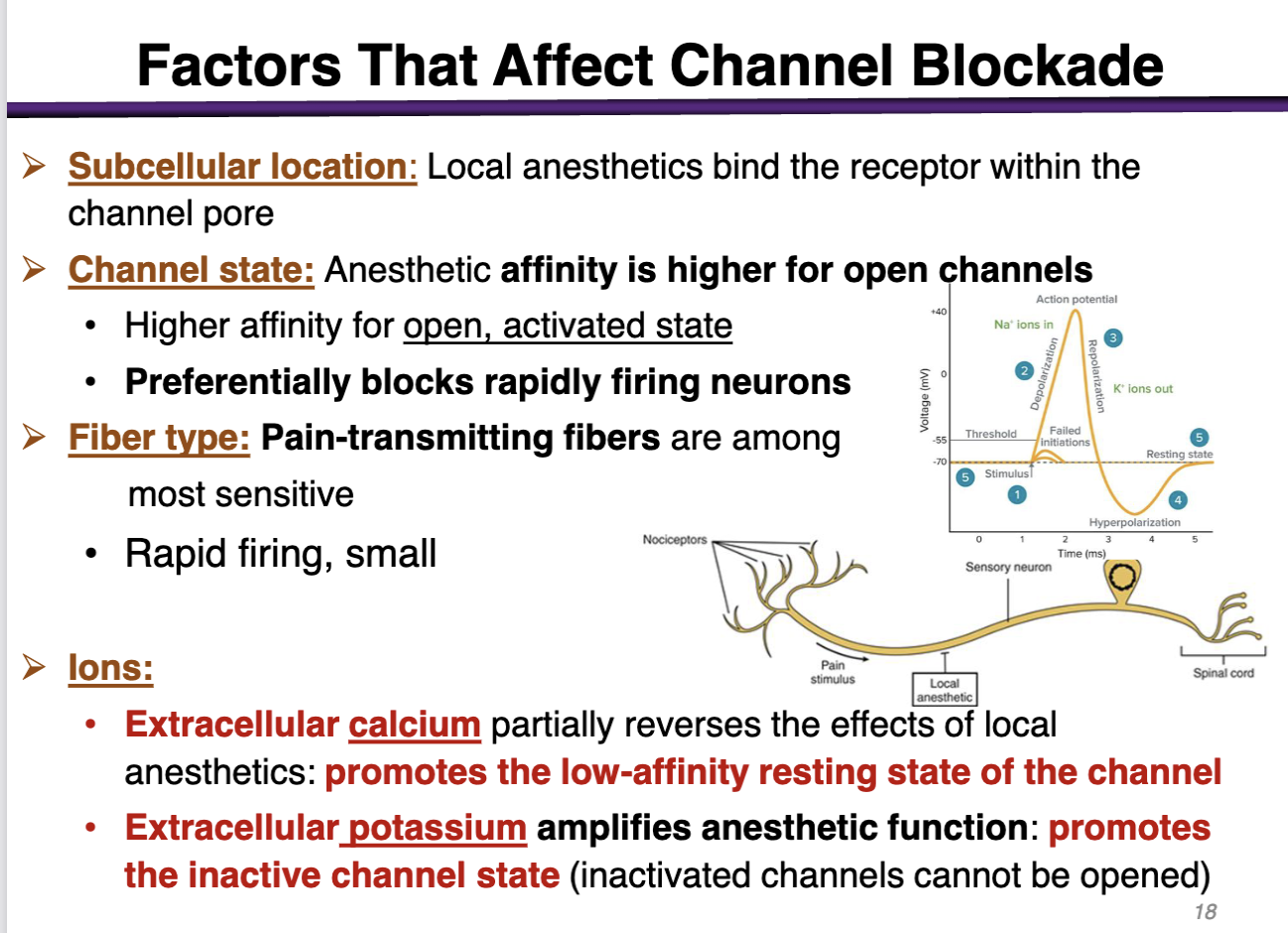
how can fiber type factor affect channel blockade
pain-transmitting fibers are among the most sensitive
rapid firing, small
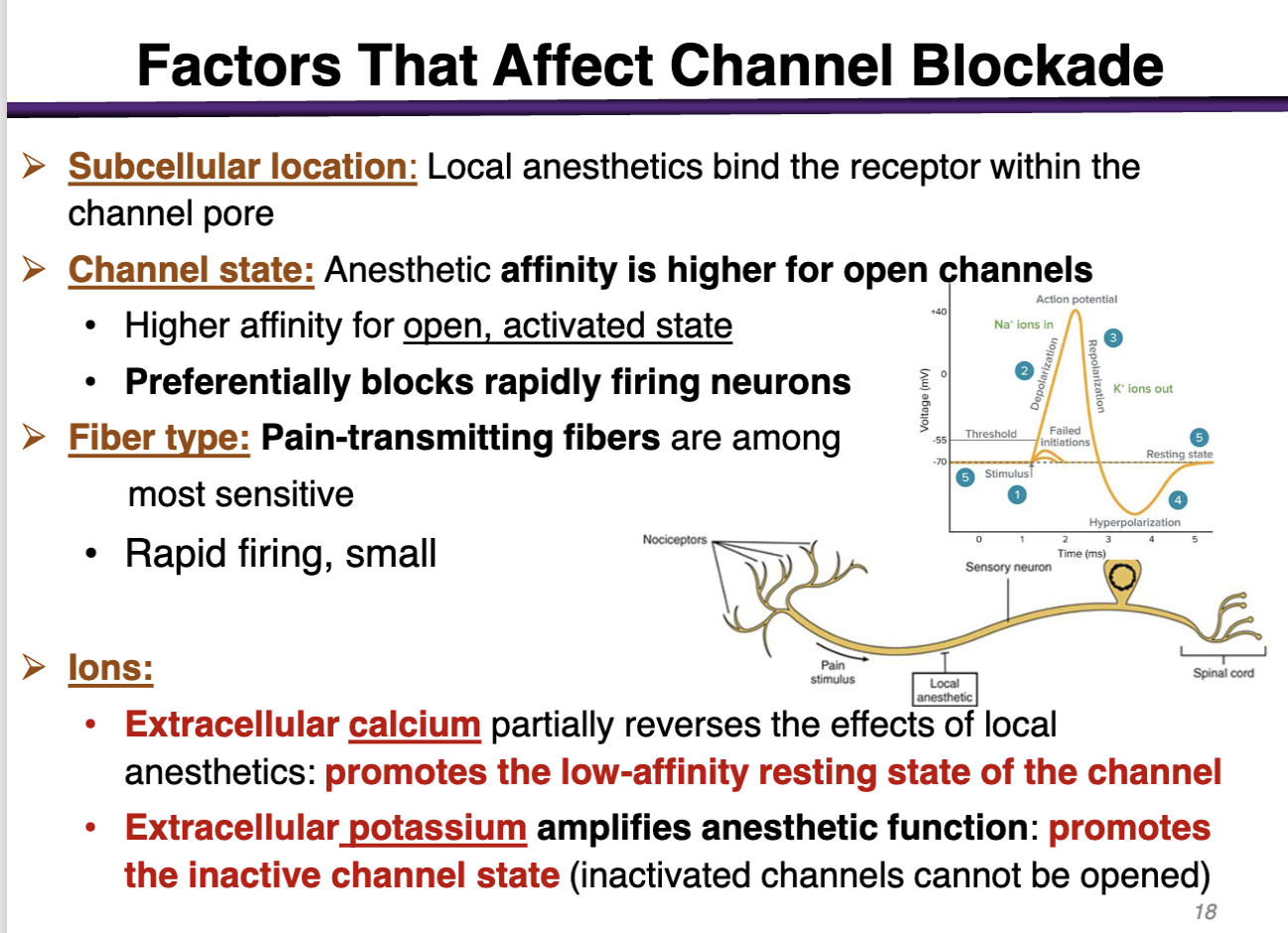
how can ions affect the channel blockade
• Extracellular calcium (+) partially reverses the effects of local anesthetics: promotes the low-affinity resting state of the channel (Not good; local anesthetics have the highest affinity for the open state, activated state)
• Extracellular potassium amplifies anesthetic function: promotes the inactive channel state (inactivated channels cannot be opened) (Good; promotes the inactivated state)
short acting local anesthetics
procain, chloroprocaine (ester)
intermediate local anesthetics
lidocaine, mepivacaine, prilocaine (amide)
long acting local anesthetics
tetracaine (ester), bupivacaine, levobupivacaine, etidocaine, ropivacaine
lipid-solubility correlates with
higher potency
drugs used for epidural administration in pregnancy
bupivacaine is NO LONGER USED
Ropivacaine and levobupivacaine are less toxic in pregnancy
clinical use of local anesthetics for neuropathic pain
lidocaine is used
neuropathic pain is caused by damage or injury to the nerves
adjuvant to tricyclic antidepressant/anticonvulsant combinations
The following anesthetics are all long-acting except
A. Tetracaine
B. Bupivacaine
C. Procaine
D. Etidocaine
E. None of the above
Procaine
Which one of these local is NOT an amide
A. Benzocaine
B. Bupivacaine
C. Mepivacaine
D. Lidocaine
E. Etidocaine
A. Benzocaine (this is an ester type)