OCHEM 2 EXAM 1 CH.1 Hybridization
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
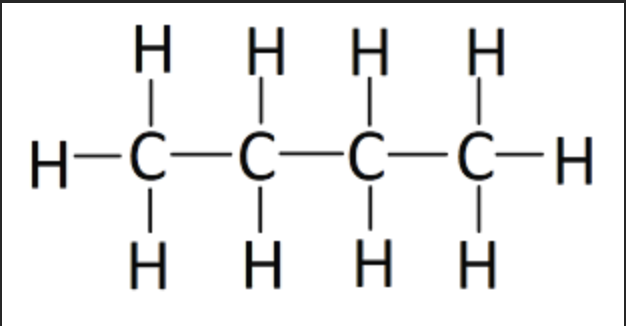
What is the hybridization of the indicated atom?
sp3 hybridized
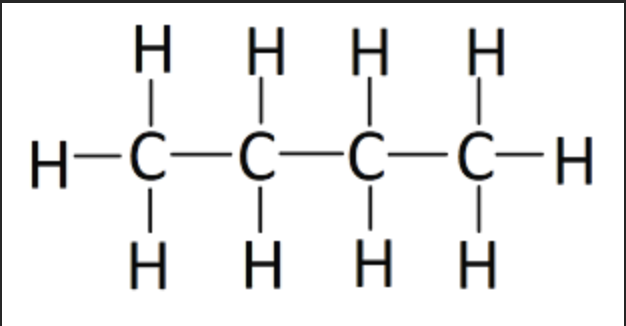
Which orbitals are used to form the covalent bonds in this atom? CH3CH2CH2CH3
The carbon-carbon bonds are formed by the overlap of an sp3 orbital of each carbon.
What orbitals overlap to form the C-H bond in ethene?
s-sp2 orbitals
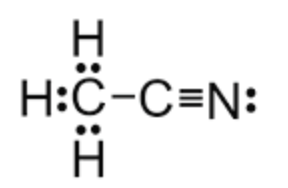
CH3CN contains how many sigma and pi bonds.
5 sigma and 2 pi bonds
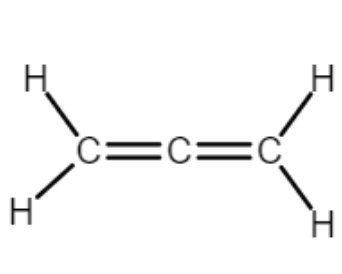
How many sp2 carbons are present in this molecule?
2 sp2 carbons
What orbitals overlap to form CH3CH3+?
s-sp2 orbitals overlap
The lone pair of electrons in a methyl anion must occupy what orbital?
sp3 orbital
regular methyl is not sp3
The N-H bond in the ammonium ion NH4+ is formed by the overlap of what two orbitals?
s-sp3 orbitals
s of H and sp3 of N
Of the hydrogen halides the strongest bond is what? What is the longest bond?
HF is the strongest
HI is the longest/weakest
What is the bond angle and hybridization of the carbon in CH3+?
120º sp2 hybridized
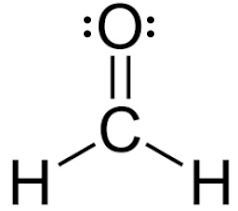
What is the hybridization of carbon in H2CO?
sp2 hybridized
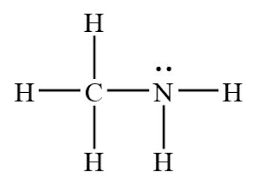
The N-H bond in CH3NH2 is a_____bond formed by the overlap of an____orbital of N and the_____orbital of H?
s bond sp3 orbital, s orbital
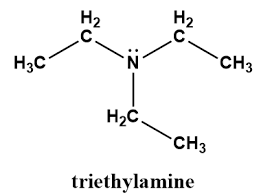
The Nitrogen atom in (CH3CH2)3N is____hybridized and the C-N bond angle is?
sp3, <109.5
Nitrogen lone pair pulls down
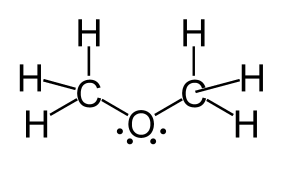
Which of the following angles is closest to the C-O-C bond angle in CH3-O-CH3?
109.5º
What is the C-N-N bond angle in the following angle?
sp2 120º
What is the hybridization and bond angles of each carbon in CH3CN?
CH3-sp3 109.5º
C-sp 180º
Which bond in the following compound is the shortest?
Triple Bond SHORTEST
The carbon carbon double bond in ethene is____and_____than the carbon-carbon triple bond in ethyne?
weaker, longer
double bond weaker and longer than triple bond, triple bond strongest and shortest
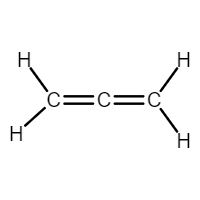
Draw the structure of a compound that contains only carbon and hydrogen atoms that has 2 sp2 carbons and one sp carbon?
Both CH2 are sp2 carbons
Central C is sp carbon
Why is the C-H bond in ethene shorter and stronger than the C-H bond in ethane?
The length and strength of C-H bond depends on the hybridization of the carbon. The more s character means the shorter and stronger the bond. Ethene uses sp2 orbitals (1/3 s character) Ethane uses sp3 orbitals (1/4 s character).
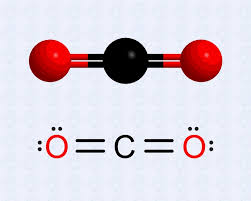
How many non-bonding pairs of electrons, bonding electron pairs, pi bonds and sigma bonds are present in CO2?
4 non-bonding pairs
2 pi bonding pairs
2 sigma bonds
What is the hybridization of the oxygen in CH3OCH3?
sp3 hybridized 109.5º
What is the hybridizations of the carbons, from left to right in CH3CHCHCl
sp3,sp2,sp2
What is the hybridization and bond angles in CO2?
sp and 180º
Which of the following has the weakest bond?
HI
What is the hybridization of (CH3)3N?
sp3
N bonded to CH3 with lone pair 4 orbitals for electrons
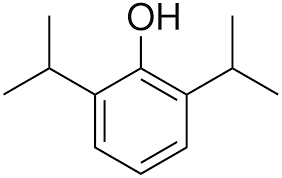
The structure of propofol is shown. How many sp3 hybridized atoms are in this compound?
isopropyl 3 sp3 carbons 6 total
Oxygen sp3 hybridized
7 total sp3 hybridized atoms
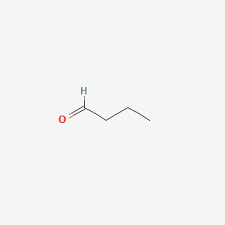
How many sp2 hybridized atoms are in the following compound?
2 sp2 hybridized atoms
How does bond strength change as bond order increases?
Increases