instrumentation exam 3
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
principles of hybridization
describe the nucleic acid chemistry of DNA
• Stores human genetic information
• Dictates amino acid sequence of peptides & proteins
• Is composed of 2 strands of nucleotides
• Strands are arranged in a double helix.
• Helical structure is stable due to many hydrogen bonds between base pairs & hydrophobic interaction between bases.
• Bonds can be broken & strands denatured & separated.
describe the nucleic acid chemistry of RNA
• Present in human cells & chemically similar to DNA
• Differs from DNA in 3 ways:
• 1) Ribose replaces deoxyribose as sugar.
• 2) Uracil replaces thymine as a purine base.
• 3) RNA is single-stranded.
• Works together with DNA to synthesize proteins
• DNA is routinely denatured, binds to RNA, & reanneals to reestablish original DNA, always following base pair rules.
the principle of DNA sequencing
• Gold standard for many molecular applications, from mutation detection to genotyping
• Requires proper methodology & interpretation
• Sequence should be analyzed on both DNA strands for greater accuracy.
• Patient sequences are compared to known reference sequences.
• Most sequencing strategies include polymerase chain reaction (PCR) amplification as first step to amplify region of interest.
• Most commonly used to detect mutations
the principle of polymerase chain reaction
• Enzymatically synthesizes millions of identical copies of target DNA to increase analytic sensitivity
• When target is microbial RNA or mRNA, RNA must be enzymatically converted to DNA by reverse transcriptase (RT).
• Latest innovation: real-time RT-PCR
• DNA melt curve analysis
• PCR: Limited by expense, need for special thermocyclers, potential aerosol contamination, nonspecific annealing & degree of stringency
the principle of transcription-based amplification system
• Detects target RNA & involves continuous isothermic cycles of reverse transcription (RT)
• Two other non-PCR methods developed from this one:
• Nucleic acid sequence-based amplification (NASBA)
• Transcription-mediated amplification (TMA)
the principle of southern blot
the principle of in situ hybridization
-is the specific annealing of a labeled probe to complementary sequences of a target nucleic acid (DNA or mRNA) in a fixed specimen, followed by detection and visualization of the nucleic acid hybrids with cytological methods
the principle of restriction fragment length polymorphism
the principle of gel technology
- The gel test, which is performed in a specially designed microtube, is based on the controlled centrifugation of RBCs through a dextran-acrylamide gel that contains predispensed reagents.
- Large agglutinates are trapped at the top of the gel and are not allowed to travel through the gel during the centrifugation.
- Agglutinated RBCs remain trapped in the gel, whereas unagglutinated RBCs travel unimpeded through the length of the microtube, forming a pellet at the bottom following centrifugation
- Agglutination reactions in the gel test graded from 1+ to 4+ (including mixed-field), just like the reactions in the test tube hemagglutination technique
- False positive mixed-field reaction interpretation
- Reaction grading considerations
target amplification
•Strand displacement amplification (SDA)
•Originally developed & patented by Becton Dickinson, Inc. (Franklin Lakes, NJ) in 1991
•One set of primers incorporates a specific restriction enzyme site that is later attacked by an endonuclease.
•Resulting "nick" created in only one strand by restriction enzyme allows for displacement of amplified strands.
•Amplified strands, in turn, serve as targets for further amplification & nick digestion.
probe amplification
•Several techniques amplify detection molecule or probe itself, instead of target.
•Ligase chain reaction (LCR)
•Uses 2 pairs of labeled probes that are complementary for 2 short-target DNA sequences in close proximity
•After hybridization, DNA ligase interprets break between ends as a nick & links probe pairs.
signal amplification
•Designed to increase signal strength by increasing concentration of label
•Employs multiple probes & several simultaneous hybridization steps
•Best-known system is branched chain signal amplification (bDNA).
•Most recent system has high specificity & can provide quantitative detection over a broad range.
•Well suited to detection of nucleic acid target with sequence heterogeneity (hepatitis C & HIV)
describe the reactions expected in gel agglutination
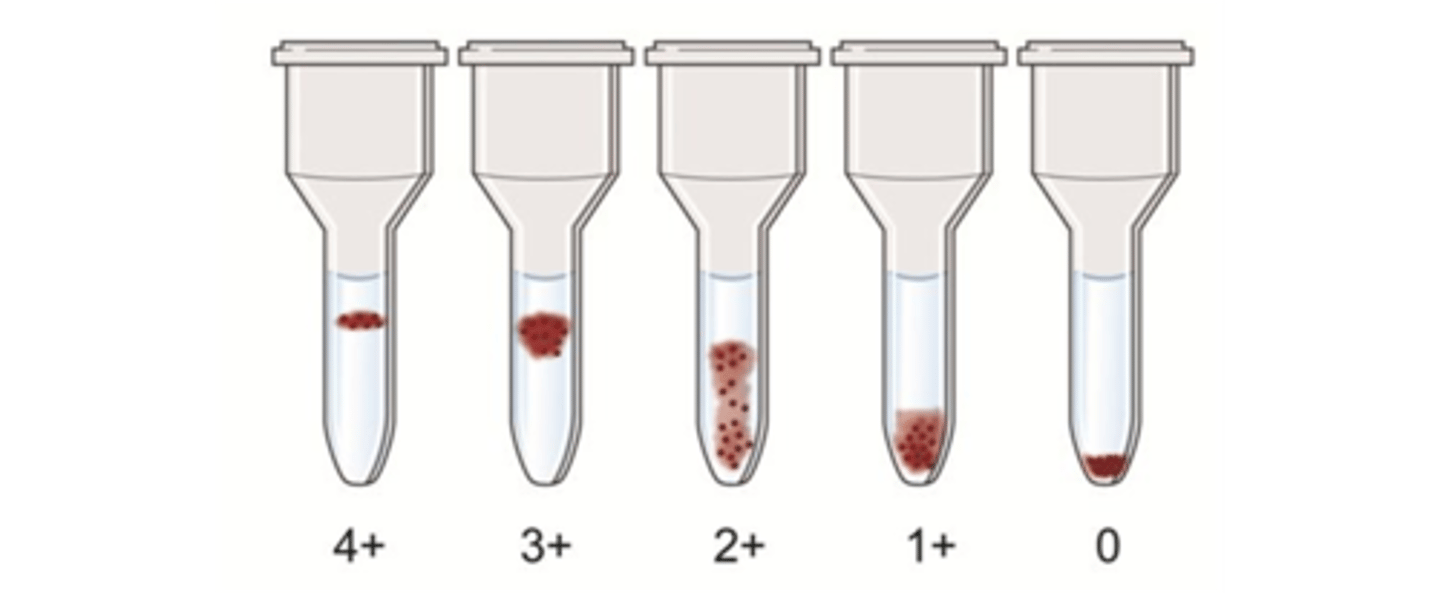
describe the advantages in gel agglutination
•Standardization of procedures
•No wash step
•No need for antiglobulin control cells
•Decreased sample volume requirements
•Enhanced sensitivity and specificity
•Improved productivity
describe the disadvantages in gel agglutination
•The major disadvantages of the gel technology are the sample restrictions and the need for special equipment.
•Hemolyzed, grossly icteric, or grossly lipemic blood samples
•Effects of Rouleaux
•Special incubators, centrifuges, and pipettes are needed
avidity
- Refers to the overall strength of the binding of antibody and antigen
- Strength of the actual bond
affinity
- Refers to the thermodynamic quantity defining the energy of interaction of a single antibody combining site and its corresponding epitope on the antigen
- "Goodness of fit"
- The original stimulating antigen is a better fit than a cross-reacting antigen
antigen
- Relatively large and complex and usually has multiple sites that can bind to antibodies with different specificities.
- Each site on the antigen is referred to as an antigenic determinant or epitope.
antibody
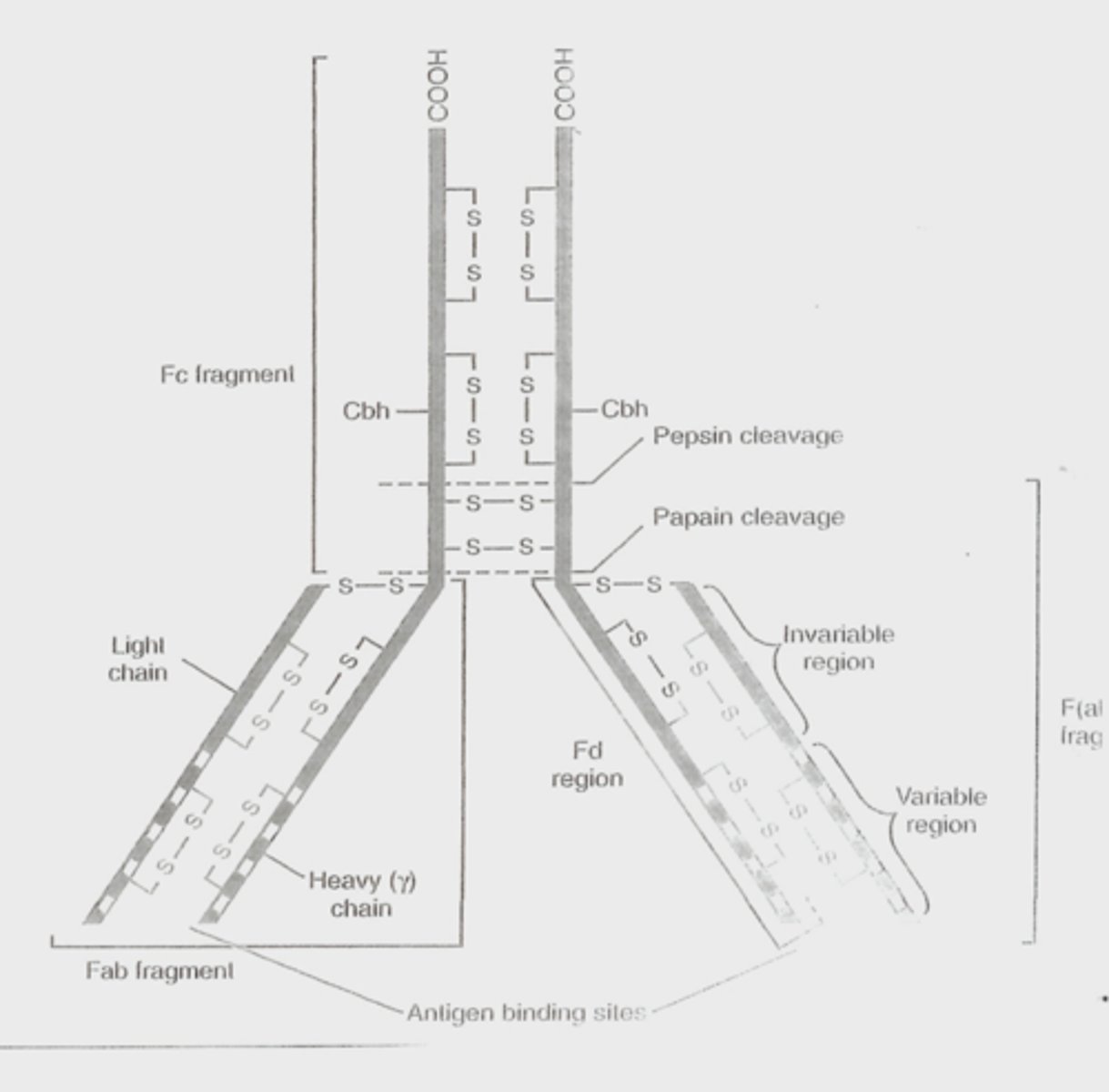
- An immunoglobulin with a functional domain known as F(ab). This area of the immunoglobulin protein binds to a site on the antigen.
- The most commonly used antibody is IgG.
- Ig G is a glycoprotein composed of two duplex chains
-- γ gamma is a heavy chain
-- κ or λ (kappa or lambda) are light chains
- The heavy and light chains are jointed by a disulfide bond
- The variable amino acid sequence at the amino terminal end of each chain determines the antigenic specificity of the particular antibody
prozone
- when antibody exists in excess
postzone
- when antigen exists in excess
zone of equivalence
- maximal precipitation or maximal lattice formation
agglutination
- the "clumping" together in suspension of antigen-bearing cells, microorganisms, or particles in the presence of specific antibodies, also known as agglutinins.
hemagglutination
- Agglutination reaction which the antigen is located on a rbc. Rbcs are good passive carrier of antigens and can also be coated easily with foreign proteins
latex agglutination
- Uses latex as an antigen carrier
describe the factors that influence antigen-antibody binding
describe the basic structure of an antibody
describe the three phases of antigen-antibody reaction and give examples of testing applicable for each phase
double diffusion
- oucherlony technique
- Agarose is used with six antibody wells surrounding a single antigen well in the center.
- Precipitin lines form if the antibody is present in the sample or standard.
- If antibody is absent no line forms
radial immunodiffusion
- Used to quantitate the antigen
- The antibody is added to the agar, wells are cut into the agar and standards and samples are placed in individual wells
- Antigen diffuses from the well in all directions, binds the soluable antibody in the agar and forms a complex ring around the well.
- The diameter of the ring is related to the concentration of the antigen present in the sample
counterelectrophoresis
- An immune precipitation method that uses an electrical field to cause the antigen and antibody to migrate toward each other.
- Two parallel lines of wells are cut into agar
-- Antibody is placed in one line
-- Antigen in the other
- Antibody will migrate to the cathode and the antigen will migrate to the anode, a precipitin line forms where they meet
- this is a qualitative test and is useful to detect bacterial antigens in CSF.
immunoelectrophoresis & immunofixation electrophoresis
- These two methods are used to characterize monoclonal proteins in serum and urine
- First the serum proteins are separated using electrophoresis
- Then reagent antibody is placed in a trough running parallel to the proteins
- The antibody reagent and proteins diffuse; when the antibody recognizes the serum protein and the reaction is in the zone of equivalence; a precipitin arc is formed.
- In IFE, electrophoresis occurs then cellulose acetate is saturated with antibody and applied to the lane of separated protein.
- If the antibody recognizes the protein, an insoluble complex is formed.
rocket electrophoresis
- Antibody is mixed with agarose gel, antigen is placed in the well and electrophoresed.
- As the antigen moves through the agarose, it reacts with the antibody and forms a "rocket"
- The height of the rocket is proportional to the concentration of the antigen
competitive immunoassay
- A radiolabeled antigen (tracer)
-- Competes with unlabeled antigen (sample) for a limited number of binding sites (antibody)
-- The labeled and unlabeled are "competing" for binding sites.
-- As the concentration of the Ag increases, more binds to the Ab, resulting in less binding of Ag*
- The Ag-Ab reaction can be accomplished in one step when labeled Ag*, unlabeled Ag, and reagent AB are simultaneously incubated together to yield
-- Bound-labeled antigen (Ag*Ab)
-- Bound-unlabeled antigen (AgAb)
-- Free-labeled antigen (Ag*)
- As the concentration of unlabeled antigen increases, the concentration of free tracer increases.
noncompetitive immunoassay
- Sometimes called immunometric assays
- Noncompetitive immunoassays use a labeled reagent antibody to detect the antigen.
- Example is Sandwich assay
-- Immobilized unlabeled antibody captures the antigen
-- Washing step performed to remove unreacted molecules
-- Labeled detector antibody is added
-- Another washing step performed to remove free labeled detector antibody
-- The signal from the bound-labeled antibody is proportional to the antigen captured.
- The sandwich assay can also be used as a noncompetitive assay to detect antibody
-- Immobilized antigen captures a specific antibody
-- After washing, the labeled detector antibody is added and binds to captured antibody
-- The amount of bound-labeled antibody is directly proportional to the amount of specific antibody present
immunoblot
- Western blot: transfer technique used to detect specific antibodies
- Multiple protein antigens are isolated, denatured, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
- SDS denatures protein and adds an overall negative charge proportional to molecular weight of protein.
- PAGE allows separation of protein based on molecular weight.
- Immunoblot (Western blot) to detect antibodies to HIV antigens
direct immunocytochemistry
- antigen is integral part of cell or tissue
indirect immunocytochemistry
- cells or tissue are used as substrate to capture serum antibody
immunophenotyping by flow cytometry
- Technique using a flow cytometer to detect intracellular and cell surface antigens
- Used to classify cell lineage and identify stage of cell maturation
- Aids in diagnosis of leukemias and lymphomas
- Begins with a living cell suspension
- An aliquot of cell suspension is incubated with one or more monoclonal antibodies (MAbs).
- If cell expresses antigen, then labeled MAb binds and fluorescent label can be detected.
compare and contrast the general types of labels used in immunoassays
classify an immunoassay, given its format, as homogeneous or heterogeneous, competitive or noncompetitive, and by its label
explain how the concentration of the analyte in the test sample is related to the amount of bound labeled reagent for competitive and noncompetitive immunoassays
describe the three methods used to separate unbound labeled reagent from bound labeled reagent
ELISA
- Enzyme Linked Immunosorbent Assays
-- Popular Heterogeneous immunoassay
-- Enzyme label and use a solid phase for separation
EMIT
- Enzyme Multiplied Immunoassay Technique
-- Homogenous assay
-- Labeled antigen and patient antigen compete for sites on the antibody
-- When labeled antigen attaches to the antibody the configuration is such that the label is inhibited, therefore only free labeled antigen is capable of producing a signal
SLFIA
- Substrate-level Fluorescence Immunoassay
-- Homogenous competitive assay
-- Antigen is labeled with a substrate
-- When catalyzed by an enzyme it will produce fluorescence
-- Bound-labeled antigen cannot be catalyzed by the enzyme
-- Only free labeled antigen can react
FPIA
- Fluorescence Polarization Immunoassay
-- Also uses a fluorescent label
-- Homogenous assay
-- Uses polarized light to excite the fluorescent label
-- Free fluorescent-labeled antigens, rotate rapidly and randomly, which interrupts the polarized light
fc fragment, fab fragment, heavy chain, antigen binding sights, light chains
example for each phase of antigen-antibody reaction
beta emission
- Nucleus can emit negatively charged electrons called beta particles or positively charged particles called positrons
- Tritium (3H) is the type most commonly used
gamma emission
- Electromagnetic radiation with a very short wavelength that originates from an unstable nuclei.
- As a radionuclide releases energy and becomes more stable, it decays, releasing energy.
- A specific spectrum of energy is associated with each radionuclide
- The half-life is the time needed for 50% of the radionuclide to decay and become more stable
- In the laboratory 125I is commonly used
- Scintillation detectors are used to detect the energy released
-- Also known as gamma counters
-- The energy released during decay excites a “fluor” (thallium-activated sodium iodide), which is amplified and detected by a photomultiplier tube
- Detectable decay is expressed as counts per minute
- One reactant, either the antigen or antibody, is radiolabeled
enzyme label example
horse radish peroxidase
describe the screening testing in coagulation
•Bleeding Time
–Manual method that evaluates primary hemostasis (being replaced by PFAs)
•Prothrombin time (PT)
–Extrinsic and Common Pathways
•Activated Partial Thromboplastin Time (aPTT)
–Intrinsic and Common Pathways
•Thrombin Time (TT or TCT)
–Conversion of Fibrinogen to Fibrin
•Quantitative Fibrinogen
–Determines amount of fibrinogen
•D-Dimer
–Detects fragments from plasmin degradation of the fibrin clot
describe the specialized testing in coagulation
•Platelet Aggregation studies
–Measures ability of VWF to support agglutination of normal platelets by Ristocetin
•Platelet Function Assay (PFA)
–Tests platelet adhesion and aggregation
•Thrombelastography (TEG)
–Real-time view of all stages of hemostasis
•Mixing Studies
– Identifies specific factor deficiencies or inhibitors
•Specific Coagulation Factors
– Determines actual activity of a factor such as Factor VIII or IX
•Antithrombin (AT or ATIII)
–In the presence of heparin, low levels of AT indicate poor clinical response to heparin
turbidometric
•Sample is added to a cuvette
•A light source is directed through the cuvette
•Initial absorbance of transmitted light is measured
•Clot initiating reagents are added by the automated instrumentation
•The plasma becomes more opaque when clotting is initiated, decreasing the light transmitted through the cuvette
•The change in transmitted light is used to calculate the result
nephelometric
•Sample is added to a sample cuvette
•The optically clear cuvette passes in front of light source
•The clot initiating reagents are added
•Light is scattered as the fibrin strands form
•The light scatters at different angles and is measured by detectors
•A clot curve is generated by consecutive readings until clot completion.
mechanical
•The sample is introduced to a cuvette that has a small steel ball inside
•The cuvette continuously moves when testing begins
•The clot initiating reagents are added to the sample
•The fibrin strands begin to form and attach to the moving ball
•An electrical circuit is either opened or closed when the ball moves away from the magnet because of the fibrin strands
•Clot time is recorded.
viscosity based
•Sample added to a cuvette with a wire or steel ball
•The steel ball or wire swings like a pendulum between two electromagnetic fields on the sides of the cuvette.
•As the plasma clots, the viscosity decreases the swinging motion of the wire or ball.
•The variation in the amplitude of this swinging motion is used to determine the clotting time
describe the use of the PFA
Platelet Function Analyzer
•Uses stimulators of platelet adhesion and aggregation in an environment that stimulates an injured blood vessel wall.
•More sensitive screening test than the bleeding time method
•Offers increased sensitivity for platelet dysfunction and von Willebrand's disease
•Nonspecific test- not diagnostic for any single disorder
•The instrument adds citrated blood to a reservoir with either collagen/epinephrine (EPI) or collagen/adenosine diphosphate (ADP) on a bioactive membrane
•A pressure sensor detects the formation of a platelet plug on the membrane
•The time it takes to close the aperture in the membrane with the platelet plug is recorded.
•The result is a function of platelet count, platelet activity, VWF activity, and hematocrit.
describe the use of the platelet aggregometry
•Performed in specialized labs by experienced laboratory professionals
•Performed on Aggregometer utilizing photometry
•Measures light transmittance over a period of time
describe the use of the TEG
•Whole Blood based analysis
•Monitors hemostasis in its entirety
-Clot initiation through clot lysis
-Measures the net effect of all hemostatic components interacting together during the clotting process
-Demonstrates the hemostatic potential of a blood sample at a given point in time.
•Sample of whole blood is placed in a cup which has a pin carefully connected to a torsion wire.
•As the cup rotates in a back and forth movement, the aggregates formed within the cup cause the wire to become more rigidly placed and reflects the strength of the aggregates formed within the cup.
•The movement or lack of movement is reflected via either an optical or magnetic detector
•A graphic presentation is produced
•Illustrates function and dysfunction in the Hemostatic system
•Allows physicians to give appropriate amounts of FFP, Cryo, and platelets to control hemorrhage
-Reduces unnecessary use of blood products
•Allows effective management of hypercoaguability
•Differentiates surgical from pathological bleeding
describe the expected results of platelet aggregometry in a von Willebrand patient
•Slowly centrifuged citrate sample yields platelet-rich plasma (PRP).
•The PRP must be adjusted with the patients PPP to reach a standard number of 200,000/µL
•The sample is stirred, warmed to 37°C in a photometric aggregometer
•The aggregating reagent (agonist) is added
-In this case, Ristocetin
•The platelets begin to aggregate which leads to a change in optical density (OD) of the PRP as measured by a absorbance detector.
•The aggregometer records the changes in OD in a graphic curve.
describe the expected results of TEG in hyperfibrinolysis, hypercoagulation, thrombocytopenia and normal patients
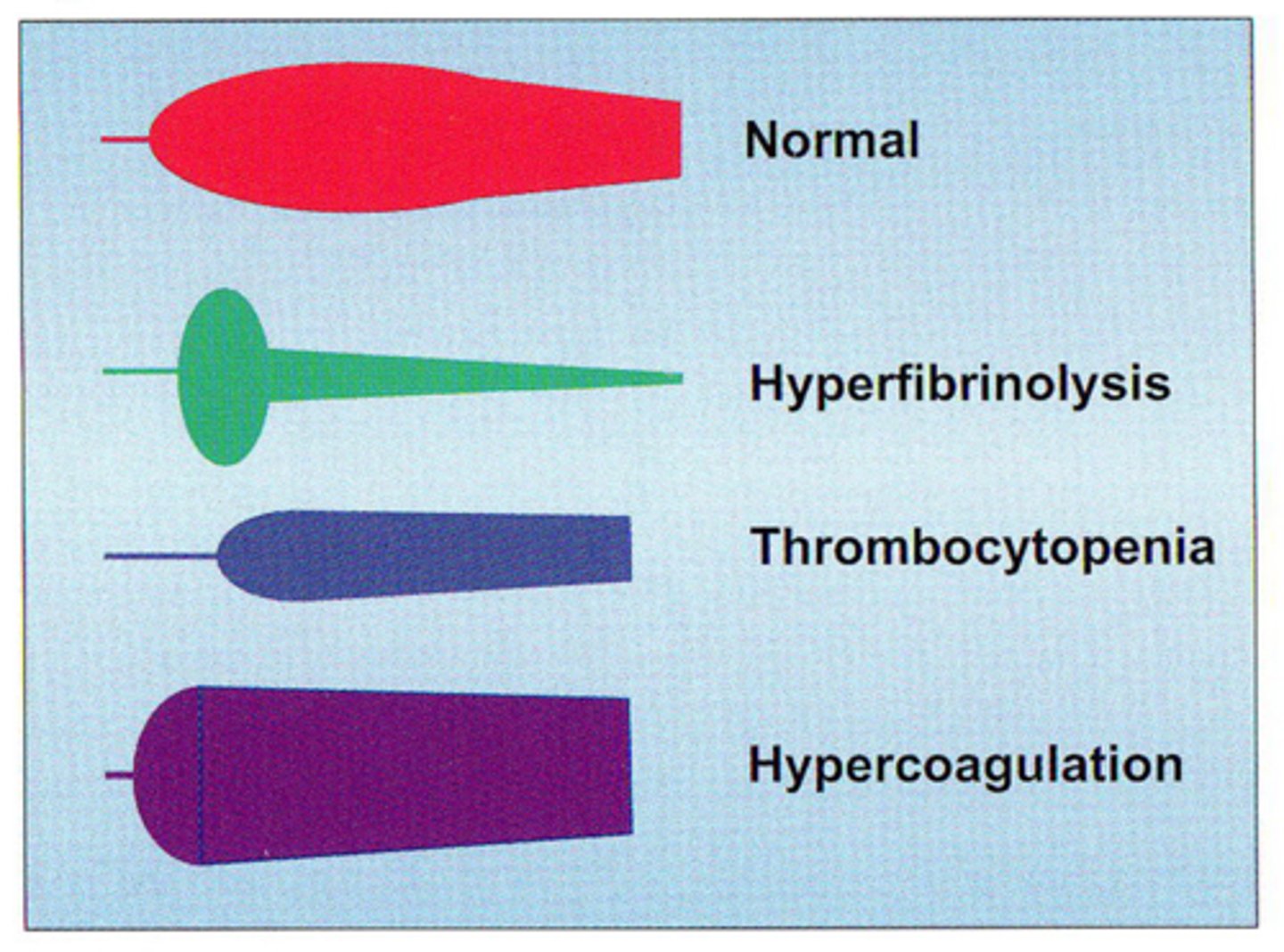
define the relationship between the maintenance of instruments and the quality of testing
Describe the preventive maintenance tasks appropriate to all analytical laboratories
- back up the system
- select the most recent event listen on the screen
- record the test count on the maintenance log
- shake down the solid waste
- with the utility routine enabled select a clean routine
- maintenance request
- enter the rack ID and press enter
- prepare and load a rack
- the system begins the clean routine
- record completed maintenance on the maintenance log
Establish a preventive maintenance program for an instrument
- Inventory
- Definition of Service Tasks
- Interval Establishment
- Personnel
- Job Assignments
- Training
- Special Instruments
- Setting Up the System
- Records & Documentation
- Surveillance
Troubleshoot instrument malfunctions using established guidelines for such activities
routine maintenance not performed
- perform routine maintenance
- repeat the calibration
problems with the pipettor, substrate, or RV wash system
- if the results of all system check routines are within the expected ranges, these systems are not the cause of the calibration failure
- if the results of any of the system check routines are not within the expected range, contact technical support for assistance in troubleshooting
reagent gone because it leaked out of the pack during off-board storage or a partial pack from another access immunoassay system was loaded on the unicel dxl system
- unload the reagent pack and load a new reagent pack
- repeat the calibration