Organic Chemistry 2 - Exam 1 (Ch. 10-11)
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
Hydrocarbon functional groups
alkane, alkene, alkyne, benzene ring
non-polar
extremely weak acids
low boiling points
only intermolecular interactions are London dispersion forces
Carbon electronegativity: 2.5; Hydrogen electronegativity: 2.2
Alkane
hydrocarbons containing no multiple bonds
substituents are called alkyl groups
common examples: methane, ethane, propane, butane, and octane
very non-polar; C-H bond is highly covalent
sp3 hybridized and have tetrahedral geometry about the carbon
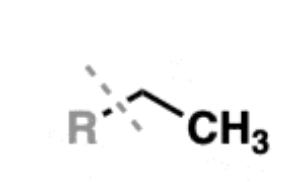
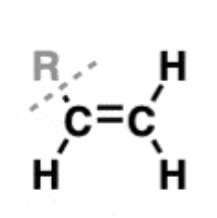
Alkene
hydrocarbons with one or more carbon-carbon double bonds
examples: ethene, propene, and butene
substituents are called alkenyl groups (-vinyl is often used to refer to -CH=CH2)
carbons are sp2 hybridized, with a trigonal planar geometry
Alkyne
contain a carbon-carbon triple bond
sometimes called acetylenes
substituents are called alkynyl groups
carbons are sp hybridized with a linear geometry
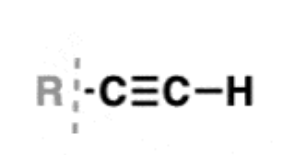
Benzene ring
six-membered ring containing 3 double bonds
aromaticity property —> makes them unusually stable
substituents are called phenol groups
carbons are sp2 hybridized with trigonal planar geometry
Amine
contains the functional group -NH2, -NHR, or NR2 (R is a hydrocarbon)
substituents are known as amino groups
examples: morphine, codeine, cocaine
amines with N-H bonds are capable of hydrogen bonding —> leads to higher boiling points and water solubility
lone pair on the nitrogen can act as a base
Alcohol
R-OH —> contains carbon bonded to the hydroxyl group -OH
examples: methanol, propanol
O-H bond is highly polarized and participates in hydrogen bonding
hydroxyl groups also increase water solubility
weak acids; can also act as Lewis bases
Ether
R-O-R —> oxygen atom flanked by two bonds to carbon
commonly used as lab solvents
examples: diethyl ether, tetrahydrofuran, dioxane
cannot serve as hydrogen-bond donor

Alkyl halides
R-X —> alkyl group bonded to a halide (F, Cl, Br, I)
examples: bromobutane, methyl bromide, chloroform
dipole-dipole interactions lead to higher boiling points than those found in alkanes
if R is alkene, they are alkenyl halides
very important functional groups for substitution and elimination reactions
Thiol (mercaptan)
R-SH
sulfur atom is not nearly as electronegative as oxygen, so the S-H bond is considerably less polarized
can act as weak acids, but stronger than alcohols
most notorious for their strong odor

Carbonyl functional groups
contain C=O bond
found in aldehydes, ketones, esters, and carboxylic acids
C=O bond is strongly polarized towards oxygen and the carbon bears a partial positive charge
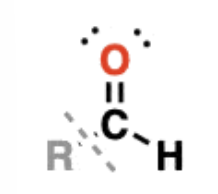
Aldehydes
RCHO —> have C=O bonded to a carbon and to C-H
examples: formaldehyde, acetaldehyde, benzaldehyde
have polar covalent bonding but are not hydrogen bond donors
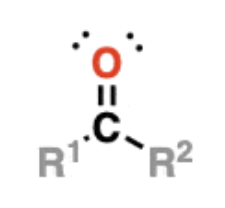
Ketones
RC(O)R —> have C=O bonded to two carbons
example: acetone
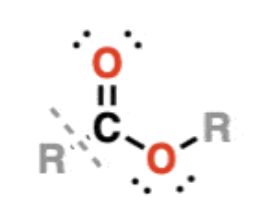
Esters
RCOOR —> similar to carboxylic acids, except the O-H bond is replaced with an O-C bond
contain polar bonds, but do not participate in hydrogen bonding
notable for their sweet smells
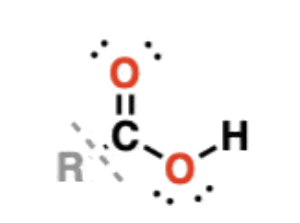
Carboxylic acids
RCOOH —> have a carbonyl bonded to -OH, but are distinct functional groups from alcohols
examples: acetic acid (vinegar), formic acid, butanoic acid
hydroxyl group participates in hydrogen bonding—> higher boiling points
tend to be relatively weak acids, not undergoing full dissociation in water
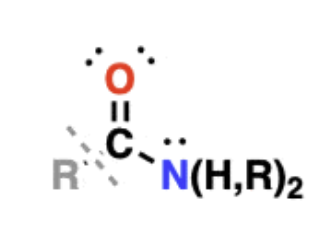
Amides
contain a carbonyl carbon attached to an amino group
amino acids linked together through formation of an amide are known as peptides
amides containing N-H bonds can participate in hydrogen bonding
Nitriles
carboxylic acid derivatives —> can be formed by dehydration of amides
common solvent is acetonitrile
-CN substituent is sometimes referred to as a cyanide; undergoes reactions with alkyl halides
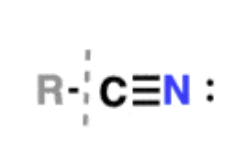
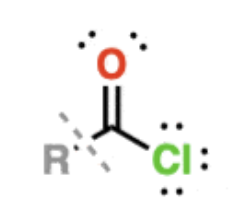
Acid halides
have -OH replaced with F, Cl, Br, or I
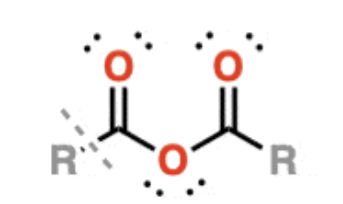
Anhydrides
contain an oxygen flanked by two carbonyls
can be formed from two equivalents of a carboxylic acid with accompanying loss of H2O
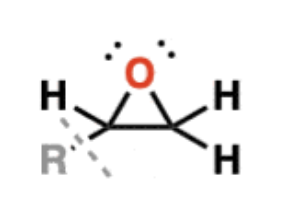
Epoxides
technically a type of ether
however, they participate in a number of reactions that ethers don’t
Spectroscopy
spectroscopy is a technique for analyzing the structure of molecules, usually based on differences in how they absorb electromagnetic radiation
four main types: nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, ultraviolet (UV) spectroscopy, and mass spectrometry (MS).
NMR spectroscopy
two types to know, 1H NMR and 13C NMR
probes the vicinity of individual nuclei, particularly hydrogens and carbons, and provides the most detailed information regarding the atomic connectivity of a molecule
Chemical shift
the difference between the resonant frequency of an isotope’s spinning protons and the signal of the reference model
in NMR spectroscopy, it is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field
the position and number of chemical shifts are often diagnostic of the structure of a molecule
Shielding
occurs when there are more electrons around the nucleus of an atom, which creates a larger opposing magnetic field
1H NMR
Gives information about:
chemical shift
integration
spin-spin splitting
13C NMR
Gives information about:
chemical shift
DEPT
Constitutional (structural) isomers
compounds that have the same molecular formula but different bonding patterns
Diastereomers
two molecules which are stereoisomers —> same molecular formula, same connectivity, but different arrangement of atoms in space
more than one chiral center —> one in same configuration, one in opposite configuration
Enantiomers
a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other
Integration
in NMR, it is a measure of the area of the peaks in the spectra
the area of the peak is proportional to the number of atoms that it represents
Coupling constant, J
a measure of the spin-spin coupling effect between two protons in a molecule
expressed in hertz (Hz) and is the difference between two adjacent sub-peaks in a split signal
to calculate, convert the peaks from ppm to hertz and find the difference
geminal coupling
the coupling of two hydrogen atoms on the same carbon atom
vicinal coupling
a type of coupling that occurs between hydrogen atoms on adjacent carbon atoms
represented by 3J, since they couple through three bonds
DEPT
Distortionless Enhancement by Polarization Transfer
used in 13C NMR spectroscopy to distinguish between a CH3 group, a CH2 group, and a CH group
Infrared (IR) Spectroscopy
Mass spectrometry (MS)
the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection
used to study and identify chemical substances or functional groups
vibrational excitation
a mechanical mechanism that causes the vibration of particles, such as HCl, during collisions with a surface
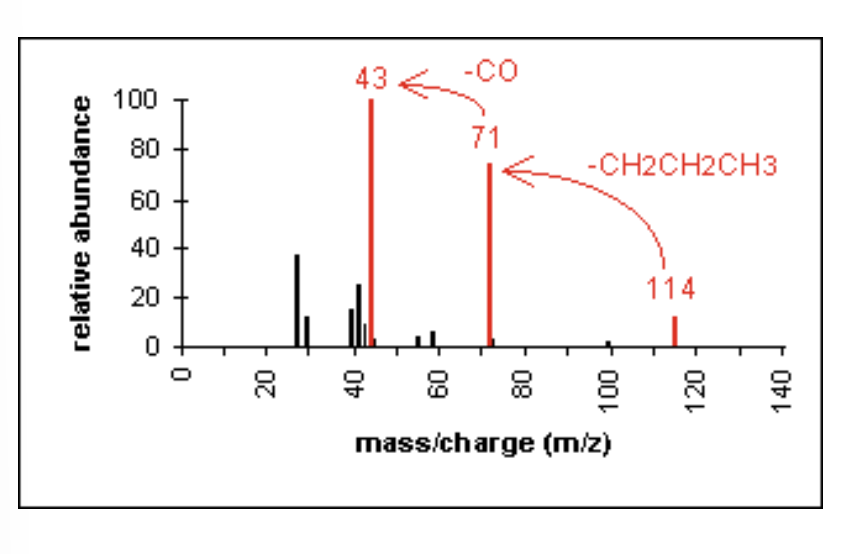
M+ molecular ion
the radical ion produced when the molecule is ionized by loss of an electron from the molecule
the m/z (mass-to-charge ratio) of this ion corresponds to the molecular weight (MW) of the sample
# of atoms x molecular weight = m/z
base peak
the tallest peak in a mass spectrum
represents the most common and most abundant ion —> most likely ion to form
assigned 100% intensity
degree of unsaturation
a calculation that determines the number of rings and multiple bonds in an organic compound
also known as the index of hydrogen deficiency (IHD)
DoU = 0 —> only single bonds, no rings
each ring or double bond counts as one DoU
triple bonds count as two degrees of unsaturation
What information is primarily obtained from nuclear magnetic resonance spectroscopy?
arrangement of carbon and hydrogen atoms in a compound
What information does the intensity (integration) of a signal in the 1H NMR spectrum give you?
A ratio for the number of hydrogens that give rise to the signal
The separation of ions in the mass spectrometer is done by their___?
Mass to charge ratio
In an IR spectrum, what does a strong absorbance peak at 1720 cm-1 indicate?
a carbonyl group, C=O
Which information is primarily obtained from infrared spectroscopy?
the functional groups present in a compound
What is the “nitrogen rule” in mass spectrometry?
organic molecules composed of C, H, O, and halogens have even molecular weights
since N forms 3 bonds, compounds with an odd number of nitrogen atoms have odd molecular weights
Resonance energy
resonance structures refers to the various arrangements of electrons among the atoms in a molecule
stretching frequency rises as bond strength and length decrease
a molecule is more stable the more resonance structures it has
Why are 13C spectra decoupled?
splitting patterns would be too complex without proton decoupling
broadband decoupling is used to suppress proton coupling and simplify the spectrum
Why is splitting not observed between adjacent carbon atoms in 13C spectra?
only 1% of carbon exists as the NMR active nuclei 13C
What is a wavenumber?
the inverse of a wavelength
What region is associated with O-H bond stretches?
3200—3600 cm-1
Geminal coupling constant
geminal hydrogens (attached to the same carbon)
0 to 2 Hz
Vicinal coupling constant
proton sets on nearby sp3 hybridized carbons
6 to 8 Hz
Cis coupling constant
hydrogen atoms in cis configuration
5 to 12 Hz
Trans coupling constant
hydrogen atoms in trans formation
11 to 18 Hz
What region corresponds to the stretching vibration of the C=C bond?
1600—1800 cm-1
Mass Spectrometry: Chlorine
isotopes: {35}Cl and {37}Cl
M+ peak will have a corresponding M+2 peak approximately 1/3 the intensity of the M+ peak
Mass Spectrometry: Sulfur
isotopes: {32}S, {33}S, and {34}S
M+ peak will have a corresponding M+2 peak that is about 4% of the intensity of the M+ peak
Mass Spectrometry: Bromine
isotopes: {79}Br and {81}Br
the M+ peak will have a corresponding M+2 peak that is approximately equal in intensity (100%) to the M+
Mass Spectrometry: Alcohol (O-H group)
typically show a loss of water (18 Da) from molecular ion
M+ peak —> can often be very small
prominent M-18 peak
cleavage of C-C bond next to the oxygen usually occurs
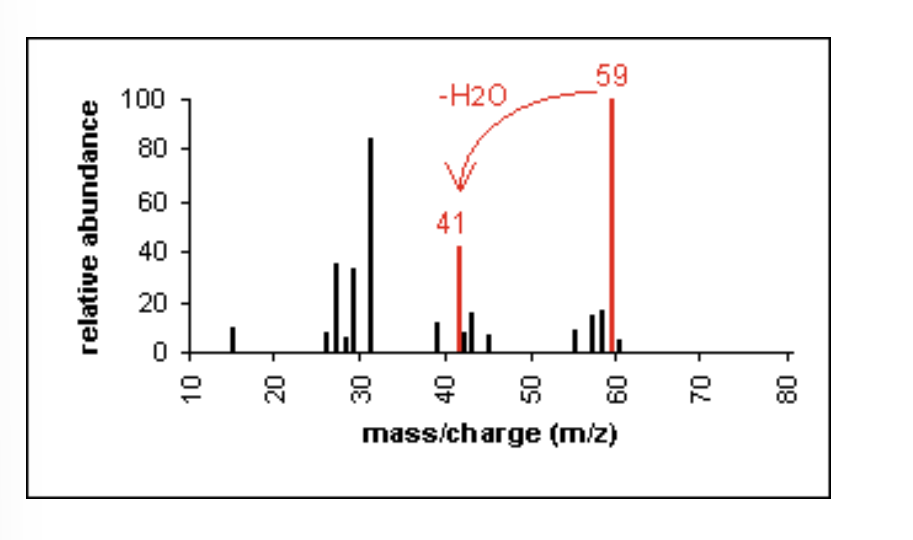
Mass Spectrometry: Aldehyde
cleavage of bonds next to the carboxyl group results in the loss of hydrogen (-1) or the loss of CHO (-29)
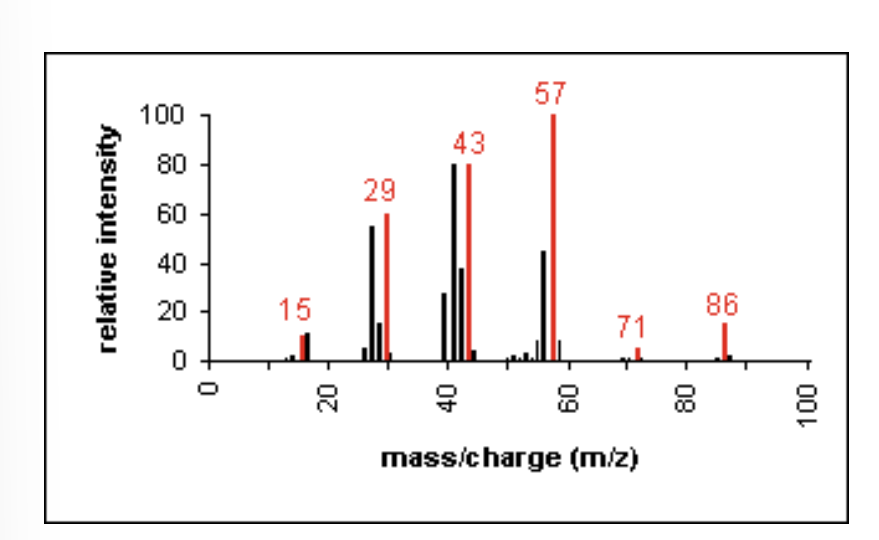
Mass Spectrometry: Alkane
molecular ion peaks are present, possibly with low intensity
fragmentation pattern contains clusters of peaks 14 mass units apart
Mass Spectrometry: Amides/Amines
primary amides show a base peak
for amines, molecular ion peak is an odd number
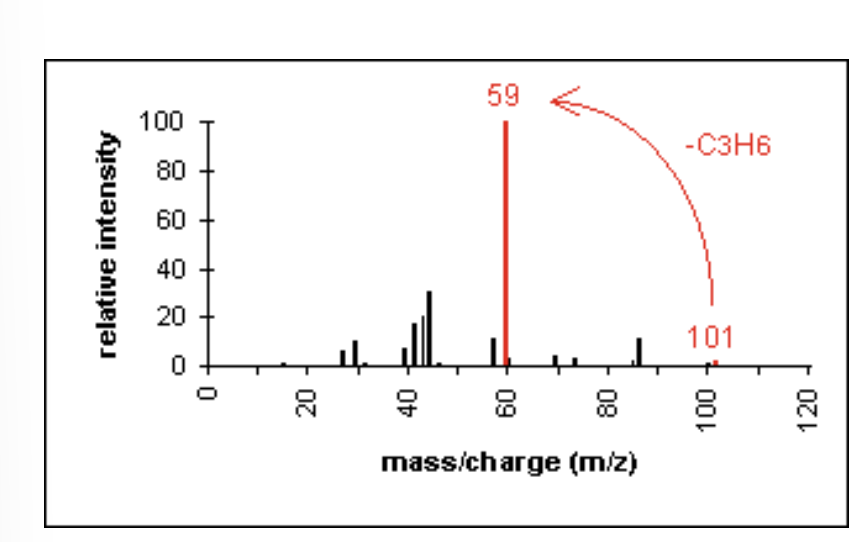
Mass Spectrometry: Aromatic compounds
molecular ion peaks are strong due to the stable ring structure
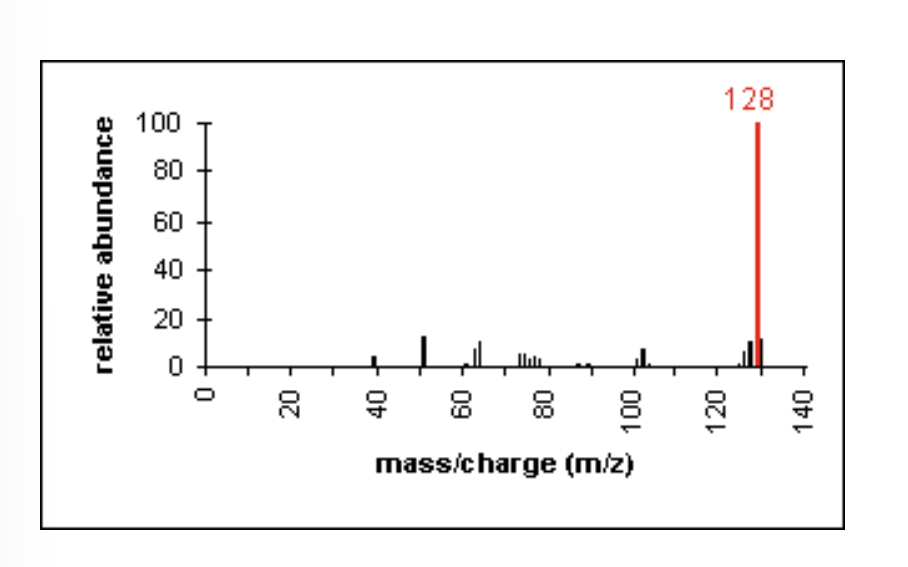
Mass Spectrometry: Esters
fragments appear due to bond cleavage next to C=O (alkoxy group loss, -OR)
also appear due to hydrogen rearrangements
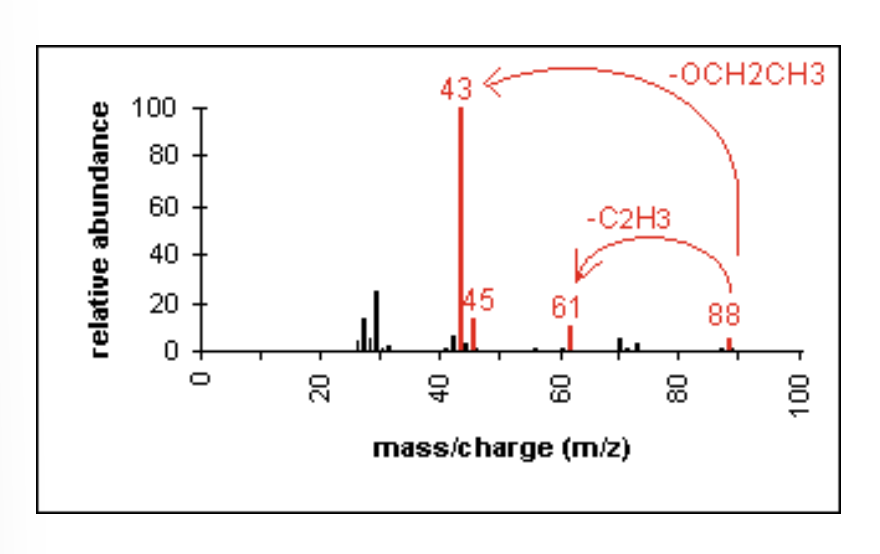
Mass Spectrometry: Ketones
major fragmentation peaks result from cleavage of the C-C bonds adjacent to the carbonyl