Batteries
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
what is a primary battery
non-rechargeable battery
what is a secondary battery
rechargeable battery
when is charge flow spontaneous
when Ecell > 0 , then G<0
what happens in Li batteries during discharging
Neutral ions intercalated at the anode are oxidised and Li ions move towards the cathode and intercalate (insert) into the cathode through the electrolyte
meanwhile electrons also flow from cathode to anode through the external circuit
SPONTANIOUS
what happens in Li batteries during charging
Neutral Li ions intercalated at the cathode are oxidised and flow back into the anode through the electrolyte.
Meanwhile electrons flow through the external circuit from cathode to anode too.
NOT-SPONTANIOUS
why are li batteries reversable
Li ions can move back and fourth between anode and cathode since they intercalate at the electrodes which is reversible
what happens at the anode of Li batteries
Li intercalated ions are oxidised and released a Li+
what happens at the cathode of a Li battery
Li ions intercalate and are reduced i
what does intercalation mean
the Li enters and leaves the ion without destroying it, but still being reduced/ oxidised
where is the energy of a battery stored
in the cathode
Really energy is stored in the difference between the electrochemical gradients of both electrodes, however, the cathode limits the energy capacity:
since it determines how many electrons can be inserted at once without damaging the structure (since cathodes are usually more fragile)
what really determines voltage
the difference in potentials between the cathode and the anode.
At the anode electrons have a higher potential and are then stabilised at the anode
If the anode has high potential the electrons are "eager to leave" then the energy will be higher, since more stabilisation (loss of energy) occurs when the electron is transferred to the anode
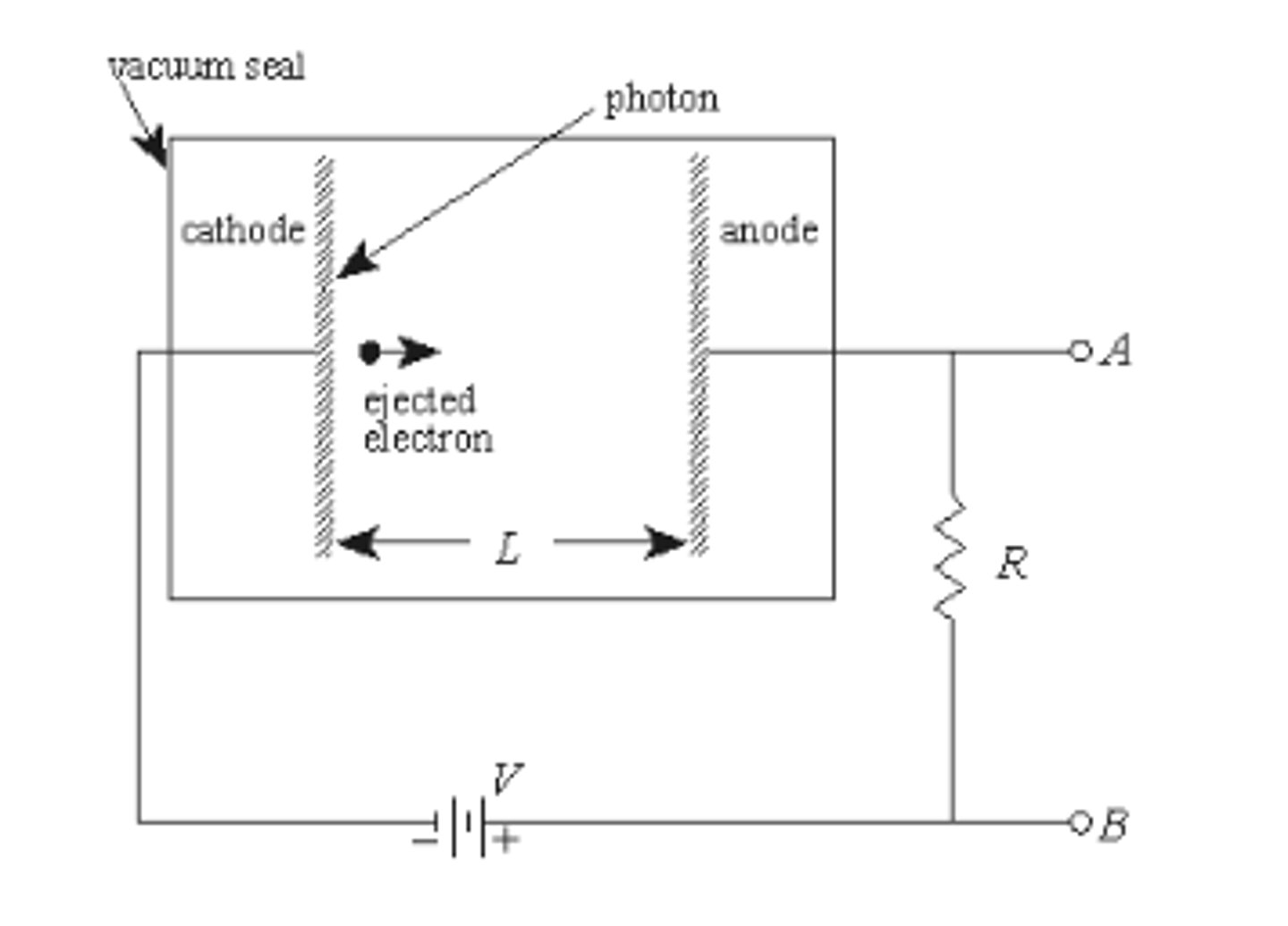
Why are cathodes more fragile than anodes?
to maintain the electrochemical gradient! They need to be made of different materials
what are anodes usually made of
graphene or something with lots of layers to accommodate Li
what are cathodes usually made of
Metal oxides LiCoO2 type thing
The metal oxides form layers that Li intercalates into,
The discovery of using these as cathodes instead of pure lithium improved safety of the devices
why do rechargeable cathodes degrade over time
the intercalation and release of Li and Li ions over time causes expansion of the material over time ,which can lead to lattice cracking and degration.
what properties are ideal for a cathode
- a high storage capacity for electrons
- good interaction w/ Li ions (to capture and release them)
- stable
what is the electrolyte
substance allowing movement of Li ions between the between electrodes
must have:
- high conductivity
- dissolves Li
- non flammable (therefore ideally not liquid at all)
what does a more negative reduction potential mean
something is more likely to give up an electron. I always think, more negative, the less it wants a bloody electron
what properties do you want at an anode
same as cathode girlie
what does higher conjugation lead to
more stable eheheh
how can pi conjugation occur
through conjugation or through space
which properties does conjugation determine
- conductivity
-light harvesting
-light emission
-electrochemical reversibility
what does pi conjugation do to the energetics of a system
provides thermal and chemical stability.
DeltaH hydrogenation is less negative (so molecule is more stable and less reactive) than expected for a molecule if it is conjugated.
Work expected value - experimental value = stability given by conjugation.
how does conjugation decrease bandgap
it both increases the energy of the HOMO and decreases the energy of the LUMO
However despite a higher HOMO the other orbitals of a more conjugated molecule are lower in energy - so the overall energy of the molecule decreases
How does conjugation lower the LUMO
The LUMO is lowered since it will be conjugated/delocalised which stabilises a system.
how does conjugation mean a higher HOMO
conjugation makes a pi orbital more stable. However - it is not more stable than a sigma bond.
For example in butane there are only sp3 sigma bonds, but in butene there are 3 x sp2 sigma bonds and 1 pi bond which will be higher in energy.
If you extendedterm-25 ethene to become lets say octene, despite the fact overall the molecule will be lower in energy, the HOMO of octene would be higher than butene, comply because octene has more orbitals, so the pi bonding oneThe LUMO is lowered since it will be conjugated/delocalised which stabilises a system. would be of a higher order.
Overall conjugation = delocalisation = more stable. But conjugation = HOMO is a pi bond = higher energy .
how does aromaticity further stabilise a conjugated system
if you look at net in phase bonding contributions for an aromatic vs an aliphatic chain, aromatics have one more net in phase bonding contribution, so therefore are more stable
How does delocalisation destabilise a molecule
spreading out the electron density or charge, thus reducing the repulsion between electrons and decreasing the overall energy of the molecule
what happens as you approach infinite aromatic conjugation e.g graphene
you end up with so many energy levels you form continuous valence and conductive bands
how do you work out exciton binding energy, Eb
Eb = e^2 / (4pi epsilon epsilon0 R0
Or Eb = Eg - Ex
Eg = band gap
Ex = energy of photon emitted from relaxation
What does a high Eb indicate
a tightly bound and hard to excite electron
for good conductivity do you want a high or low Eb
a low Eb, you want e- and h+ separated for as long as possible
what happens when you excite an electron across Eb.g
it creates a hole, h+ in the valence band and an electron in the conductive band
why can electrons conduct easier in the conductive band
more energy and also more free space to move around that (empty) band
what happens in the main principle of photovoltaic devices
an electron is excited from a material by light, and hole travels to the anode and the electron travels to the cathode
how do we overcome the high Eb of organic semiconductors, to overcome the coulombic potential of the electron and hole to instantly recombine after excitement
using a donor acceptor model. Have the electron excited from a HOMO to the LUMO of a donor molecule, then the electron jump to a lower LUMO of an acceptor molecule
the hole in the donor and the electron on the donor can then move respectively through the donor and acceptor material to the electrodes
how can on the surface of photovoltaic devices, where electrons are excited, the donor-acceptor interactions be maximised/ electron hole recombination be minimised
by maximising the surface area of of the donor/acceptor interface. (e.g by bilayer, interdigitated or bulk heterjunction methods)
what are the only UV visible electronic orbital excitation transitions
pi -> pi*
n.b -> pi*
since these are lower energy transitions >250nm
how do we maximise the solar harvesting of a molecule
we want to make conjugated molecules with such small bandgaps that they absorb in the lower energy solar/ redish light regions
what is the ionisation potential, IP
the energy needed to excite an electron from the HOMO off to the moon
what is electron affinity, Ea
the energy needed to excite an electron from the LUMO off to the moon
what is blue shift
shifting to a lower wavelength
what is red shift
shifting to a longer wavelength
hyperchromic shift
to a higher intensity absorption
hypochromic shift
to a lower intensity absorption
how do you work out λonset
draw a tangent from the longest wavelength side of the longest wavelength absorption peak of a cluster, and where the tangent intersects the x axis is the λonset
how do you work out Ebg.optical from λonset
= hv = hc/λ
h = plank's constant ( Js)
Ebg = in eV
C = speed of light nms^-1
what is the simplified subbed in version of the equation for Ebg.opt
Ebg.opt = 1241 eVnm / λonset (nm)
how does increasing conjugation effect absorption spectra
red shift
how does planarisation effect the absorption spectra of a molecule
it increases conjugation therefore red shifts
how does regioselectivity effect the band gap of a molecule
more regioregular backbone = polarised system = encouraged conjugated = smaller bandgap
what is an auxichrome
a non-photoactive substituent that changes the light absorbing properties of a chromophore
What do electron withdrawing substituents do to the bandgap/ spectra of a molecule
they increase Eg, blue shift
However if there is strong donor-acceptor interactions it can decrease the bandgap
(they stabilise HOMO, as you take away electrons, you take away the energy levels from the HOMO = higher band gap)
what do electron donating substituents do to the bandgap/ spectra of a molecule
they decrease Eg, red shift
(they destabilise the HOMO, as more electrons = more electron energy levels = higher ones = lower band gap)
what are n-type acceptors
negative type electron acceptors
how do we enhance the electron affinity of electron acceptors
we stabilise the LUMO (making the transition from donor LUMO to acceptor LUMO even more energetically favourable)
what the **** is an FMO
frontier molecular orbital i reckon
how does electrochemistry use heterogeneous electron transfer to achieve reduction
electron transfer happens at the surface of an electrode that supplies an under/ over potential for oxidation/reduction to supply the required energy for transfer
what value of applied potential do you need for spontaneous reduction
V applied > E LUMO
and vise versa for oxidation
what are the three components of a three electrode setup
-reference electrode
-counter electrode
-working electrode (where the voltage is applied)
what happens as molecules diffuse away from an electrode
they won't react as less voltage will be applied the further it moves from the electrode
what is shown in linear voltammetry
current (A) on y axis, potential on x axis
sweep the applied voltage in a single direction between two potentials.
Used to determine the half potential, E1/2 for an electron transfer
also used to determine relative no. electrons transferred in multiple transfer processes
how can you work out relative number of electrons transferred from linear voltammetry
the intensity of the peak
For a linear voltammetry of Fc/Fc+ relative to Fc/Fc+ at what potential would E1/2 be
0 of course relative to itself, there'll be no difference/ shift
on a voltammetry a sweep towards which direction indicates which process
to negative potential = reduction
to positive potential = oxidation
what does a time against potential between two potentials plot look like for linear voltammetry
a positive gradient straight line
why is E1/2 a key value
it is always constant, them middle potential between two potentials.
Despite the fact the potentials themselves may vary experimentally
what happens in cyclic voltammetry
sweep back and fourth between two potentials
how do you calculate the E 1/2 on linear voltammetry
E1/2 = (Ered - Eoox) / 2
where E red and Eox are the peaks of the respective reduction and oxidation peaks
what does a less negative reduction potential .potential mean about a molecule
that it is harder to reduce / easier to oxidise (think of it as being negative means not wanting electrons - it wants to be oxidised, not reduced typically, an anode would have very negative reduction potential, since its being reduced)
what does a time vs potential plot look like for cyclic voltammetry between two potentials
two lines converging as you go towards more negative potential
How can you tell from cyclic voltammetry if a redox reaction is reversible
if it is reversible then delta E
otherwise known as (Ered - Eox) = about 59 n (Mv)
n = no. electrons in the process
REMEMBER THIS
NEGATIVE POTENTIAL MEANS IT DOES NOT WANT ELECTRONS
how do you determine energy of the HOMO/ LUMO from cyclic voltammetry
Ehomo/lumo = -n ( Eonset ox/red - E1/2 vs Fc/Fc+ ) + IP
IP = ionisation potential of Fc = 4.8 eV
The -E1/2 of Fc thing is to set the equation with Ferrocene as the standard/ reference electrode. For going from vs Ag/AgCl to Fc/Fc+ then -E1/2 vs Fc = 0.4
so youd do Eonset - 0.4
how do you find onset of ox/red from a cyclic voltammogram
look at notes honestly i can't explain but you read it off the graph
How can you find the LUMO from only knowing the energy of the HOMO and vise versa
using UV data to calculate the band gap energy
what happens on a molecule if there are multiple reduction/oxidation sites that are conjugated to each other
Reduction/oxidation is stepwise instead of simultaneous.
Reduction/oxidation potentials are dependant /altered by any previous reductions/oxidations in the stepwise process.
e.g after each reduction the molecule will require more energy to achieve another reduction due to already increased electron density of the system.
what will a cyclic voltammogram of a molecule that has multiple reduction/oxidation sites that are conjugates vs not conjugated look like
the conjugated one will show a peak per reduction/oxidation site, each with equal intensity.
The non conjugated molecule will only show one more intense peak for all the reductions/oxidations at the same potentials since they are simultaneous processes
how do you engineer a material to have a higher IP (easier to ionise/oxidise)
use electron donating substituents to destabilise the HOMO (if you make something more electron rich its going to be easier to oxidise)
how can you engineer something to have a lower LUMO/ become easier to reduce (have a less negative onset of reduction)
at electron withdrawing substituents
what is the limit with adding electronic effecting substituents to influence the onsets of reduction and oxidation
the effects of substituent groups aren't summative
in a cyclic voltammogram which process corresponds to the energy of which orbital
oxidation corresponds to HOMO energy
(higher HOMO = more favourable oxidation)
reduction corresponds to LUMO energy
(lower LUMO = more favourable reduction)
what is through space intramolecular electronic couplign
redox sites can communicate if close in space as orbitals close in space overlap causing conjugation/delocalisation
This leads to stepwise reduction/oxidation
how do you work out Ecell
Ecell = Eoxidation + Ereduction
how do you work out the deltaG cell
= -nFEcell
n = number of electrons transferred
F = faraday's constant
what are the SI units for V
J = V C^-1
why are organic molecules bad for being intercalation electrodes
they pi or aromatic stack too densely and don't allow for the bulk ion flow
what is the advantages of using inorganic cathode materials
- defined porous lattices for bulk ion transport
- thermally/ electrochemically stable (resistant to decomposition under high rates C rates so have longer lives)
- insoluble in electrolyte which prevents them from leaking
what are disadvantages of inorganic cathodes
- limited resource
- heavier/ toxic/ less flexible
- only so many materials to tune properties of/ design with
what are the positives of using an organic cathode
- sustainably sourced/ cheap
- light/ portable/ flexible
- structurally diverse / more isomerisable
what are the disadvantages of using an organic cathode
-poorer solid state electrochemistry C-rates
- densely packed assembly prevents bulk ion flow
- low thermal and mechanical resistance makes them more likely to leach
current
I = W/ V
(in A)
C -rate (operating rate of a battery) (in units of hours^-1)
C rate = 1 /t
or amp hours/ hours
how do you work out Qtheory (battery capacity) in (mAh g^-1) or (Ahg^-1)
Q theory = n x F / Mr
n = no.electrons stored in a molecule
F = faraday's constant
mr = molecular weight
how do you work out Amp hours
Amps x hours
or mA x hours
what is Qspecific
the specific battery capacity/ how much energy the battery can provide experimentally
what are all the ways to stabilise a LUMO to enhance the kinetic stability of an electron transfer
- extend pi conjugation
- add -M substituents
- heteroatoms
-enhance electron delocalisation through space
why does stabilising the LUMO by extending conjugation shoot your battery in the leg
as you extend the conjugation, you increase molecular weight, lowering Q overall.
However if you also add more redox sites, increasing n, this can balance out
on a discharge curve how do you find Qsp and Eted
Qsp = tangent through the x axis from where curve tails off
Ered = tangent through the y axis from the plateau of the curve
why does Qsp decrease as cycle number increases
organic batteries are unstable and degrade over time