Exam 5
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
122 Terms
difference btw fertility and productivity
soil nutrients (fertility) are only one factor of several environmental conditions that affect plant growth: Water, Sunlight, Temperature, Soil depth, Soil microbial community, Soil salinity, and pH
So a fertile soil may not be productive if other factors are limiting plant growth
Arnon's Criteria of Nutrient Essentiality
1. A lack of the element makes it impossible for the plant to complete a vegetative or reproductive stage of development.
2. A deficiency can be prevented or corrected by supplying the element and is not replaceable by another element.
3. The element must be directly involved in the nutrition (metabolism) of the plant apart from correcting microbiological or chemical conditions in the soil
macronutrients (9)
C -H -O (from enviroment/atmosphere)
N-P-K
S-Ca-Mg
Carbon: form taken up by plant & use in plant?
CO2, cell wall component, carbohydrates
Oxygen: form taken up by plant & use in plant?
O2, H2O, CO2, carbohydrates
Hydrogen: form taken up by plant & use in plant?
H2O, carbohydrates
Nitrogen: form taken up by plant & use in plant?
NO3-, NH4+, proteins, amino acids, chlorophyll
Phosphorous: form taken up by plant & use in plant?
H2PO4-, HPO42-, Nucleic acids, ATP, ADP
Potassium: from taken up by plant, use in plant
K+, catalyst, ion transport, stomata regulation
Sulfur: from taken up by plant, use in plant
SO42-, SO2, amino acids, Chloroplast (photosynthesis)
Calcium: from taken up by plant, use in plant
Ca2+, cell wall component, ion transport
Magnesium: from taken up by plant, use in plant
Mg2+, central part of chlorophyll
Boron: from taken up by plant, use in plant
H3BO3, cell wall, metabolism of amino acids, flowering
Cobalt: form taken up by plant & use in plant?
Co2+, N2 fixation
Chloride: form taken up by plant & use in plant?
Cl-, osmotic adjustment in roots
Copper: form taken up by plant & use in plant?
Cu+, Cu2+, coenzyme or cofactor, photosynthesis
iron: form taken up by plant & use in plant?
Fe2+, Fe3+, coenzyme or cofactor, photosynthesis
Manganese: form taken up by plant & use in plant?
Mn2+, coenzyme or cofactor, photosynthesis
Molybdenum: from taken up by plant, use in plant
MoO42-, N2 fixation, conversion of NO3- to protein
Nickel: from taken up by plant, use in plant
Ni2+, coenzyme
Zinc: from taken up by plant, use in plant
Zn2+, coenzyme or cofactor, DNA transcription
Other kind of important nutrients
Chromium- may be required by fruit.
Silicon- very abundant in soil, may be taken up in small amounts and strengthen cell walls. Required by sugarcane.
Sodium- may replace K in some C4 plants, may be required in sugarbeets.
Vanadium- may be required by some N2 fixing plants, may substitute for Mo in some plants
three ways nutrients reach surface of roots
Mass flow (advection): movement of dissolved nutrients with water flow.
Diffusion: Movement of nutrient from higher to lower concentration. Root uptake reduces concentration near root surface.
Interception: root growth into a nutrient pool (such as a CaCO3 nodule or a bit of decaying organic matter).
Describe: foliar nutrient uptake
-A technique of feeding plants by applying liquid fertilizer directly to their leaves
can be a means of rapid nutrient supply, especially when soil nutrient availability or root activity is reduced.
the plants absorb the nutrients through the epidermis (the outer layer of cells that covers all external parts of the plant) or through the stomata (pores mostly on the underside of the leaves that regulate water content and gaseous exchanges)
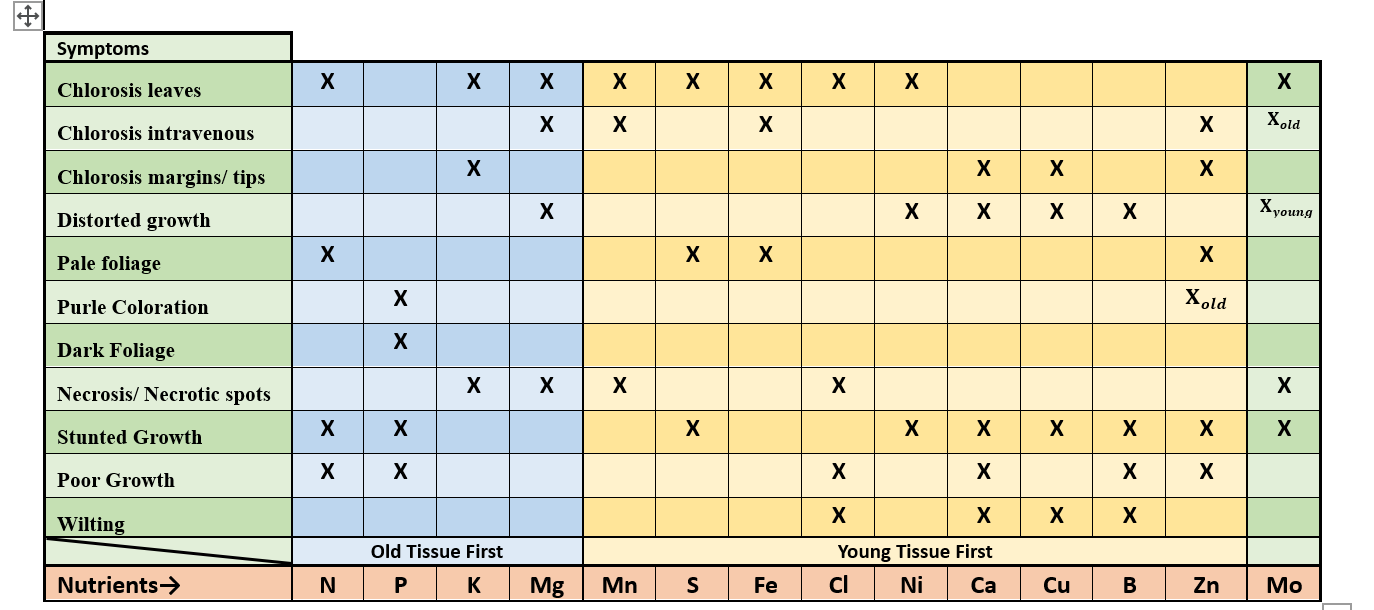
Describe: how a mobile nutrient can behave in a plant
Nutrient can relocate from one part of the plant to another, deficiency in old growth first.
Describe how: an immobile nutrient behaves in a plant
Nutrient does not move from one part of the plant to another. Deficiency will appear in new growth first.
Describing symptoms: Generalized
Symptoms not limited to one area of the plant, but rather spread over the entire plant
Describing symptoms: necrosis
Death of plant tissue; tissue browns and dies. May occur at leaf tip, margin, or interveinal.
Describing symptoms: localized
Symptoms limited to one leaf or one section of the leaf or plant
immobile nutrients (8)
Ca, B, Cu, Zn, Mn, Fe, S, Ni
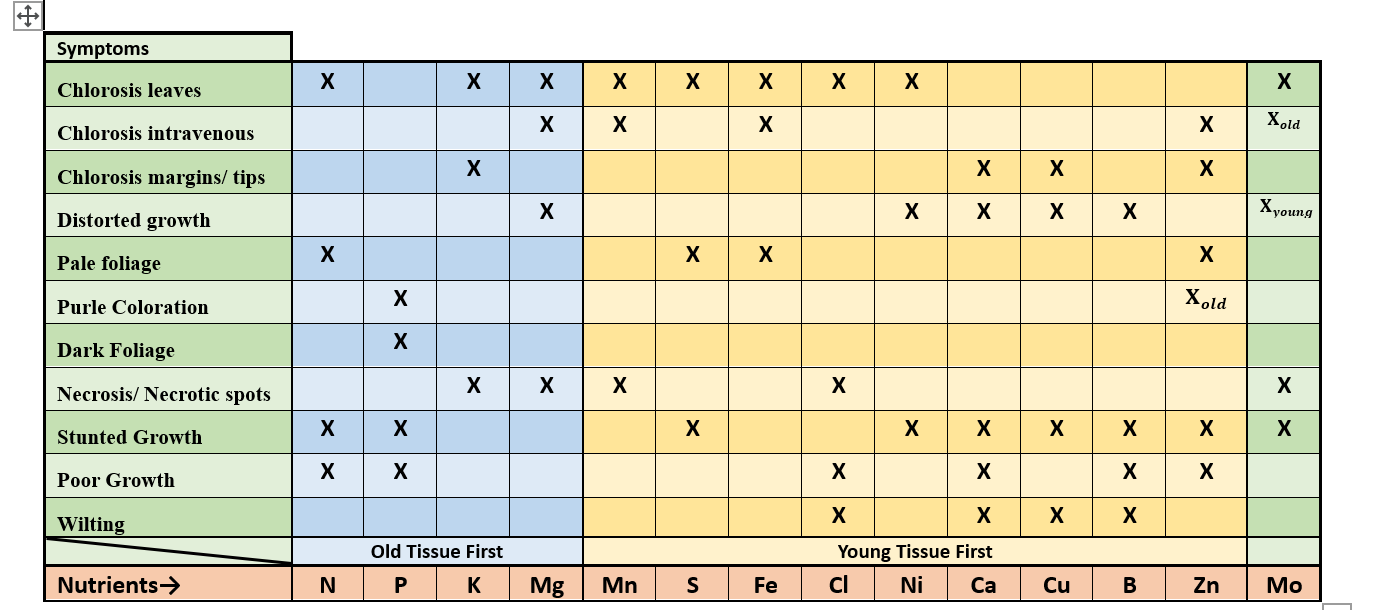
Mobile nutrients (6 )
N, P, K, Mo, Mg, Cl
Symptoms: calcium deficiency, nutrient soucre/fertilizer, etc
New leaves are distorted or hook shaped. The growing tip may die. Contributes to blossom end rot in tomatoes
Fertilizer: Gypsum or other calcium containing compound
Not often a deficiency problem and too much will inhibit other nutrients
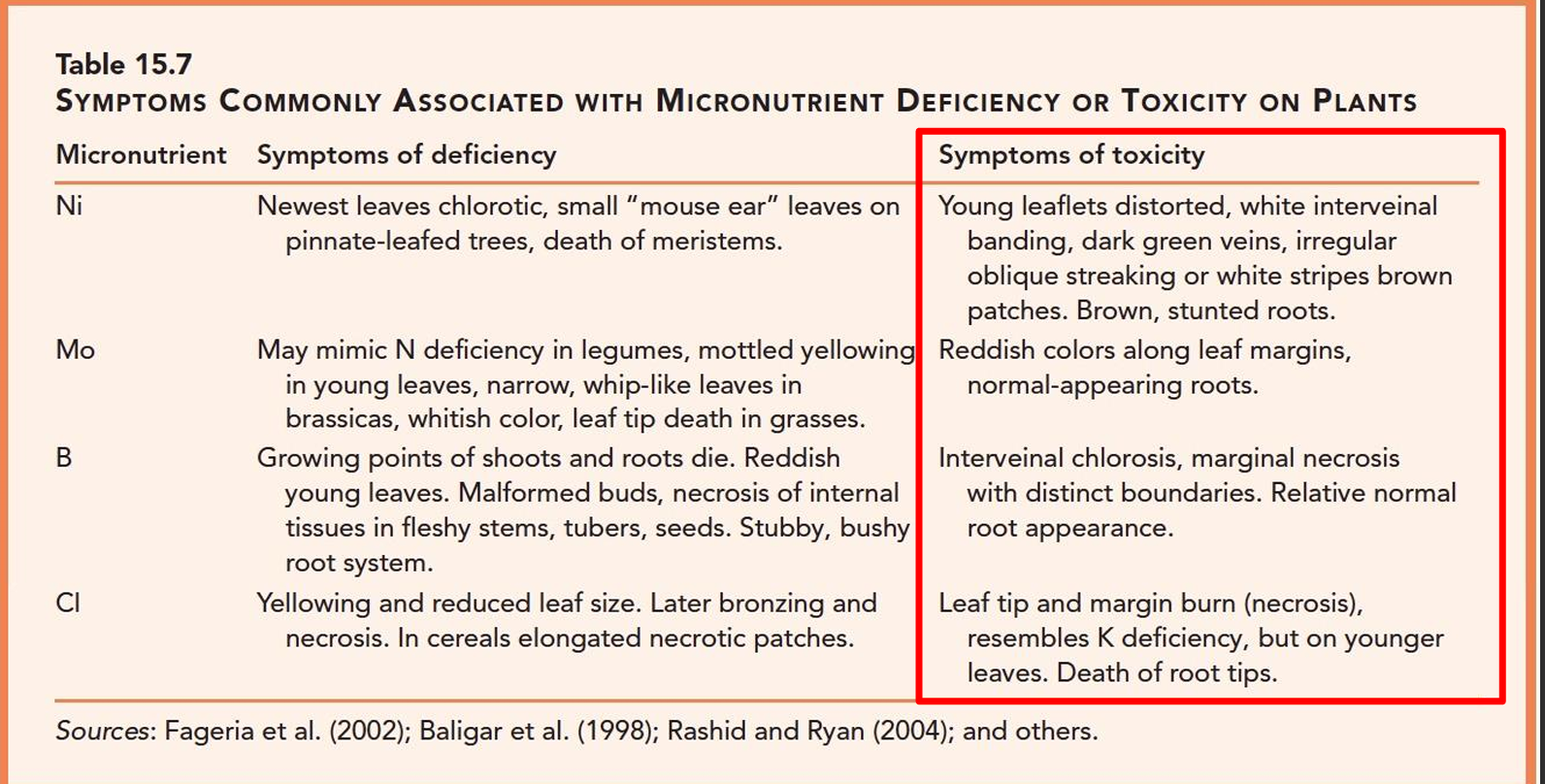
Symptoms: Boron deficiency, source/fertilizer,
Poor stem and root growth. Terminal (end) buds may die, chlorosis on new leaves (immobile), black spots on vegetables may look like insect damage
Most commonly deficient micronutrient in forest soils
Toxicity: interveinal chlorosis, necrosis with distinct boundaries
Symptoms: Copper deficiency, cource/fertilizer,
Stunted growth. Leaves can become limp, curl, or drop. Seed stalks also become limp and bend over.
Source: CuSO4·5H2O (solid)
High organic matter reduces availability and availability decreases 100x for each pH unit increase.
Symptoms: zinc deficiency, source/fertilizer
Similar to Fe, interveinal whitish chlorosis on new leaves (immobile). Terminal (end) leaves may form a rosette
Source: ZnSO4 (solid)
Common deficiency in Colorado. Availability decreases 100x for each pH unit increase.
Symptoms: Manganese deficiency, source/fertilizer
Growth slows. Younger leaves turn pale yellow (immobile), often starting between veins. May develop dark or dead spots. Leaves, shoots and fruit diminished in size. Failure to bloom.
Source: MnSO4 (solid)
Greatly affected by oxidation-reduction conditions.
Symptoms: Iron deficiency, fertilizer
Immobile in plant, interveinal chlorosis begins on new leaves, striping in grasses and corn. Iron availability decreases 1000x for each unit increase in soil pH, common deficiency in Colorado
Source: Chelated iron fertilizer, foliar application of 1-2% FeSO4 solution
Common deficiency in basic soils
Symptoms: Sulfur deficiency, source/fertilizer, etc
New growth turns pale yellow, older growth stays green. Stunts growth
Source: Sulfur or sulfur containing compounds
deficiency more prevalent in dry weather
(deficiency not common in Colorado, usually in sandy, low Om soils with high rainfall)
Symptoms: Nickel deficiency, toxicity, etc
Newest leaves chlorotic, death of meristems
Toxicity: young leaflets distorted, white interveinal banding, dark green veins, brown stunted roots
Symptoms: Nitrogen deficiency, source/fertilizer, etc
Older leaves, generally at the bottom of the plant, will yellow, often along mid-rib. Remaining foliage is often light green. Stems may also yellow and may become spindly. Growth slows.
Fertilizer: 'nitrate', 'ammonium' or 'urea'. Also manure.
Mobile in plant so deficiency first evident in lower (old) leaves, eventually spreading to entire plant.
Symptoms: Molybdenum deficiency, source/fertilizer, toxicity, etc
Older leaves yellow or mottled, remaining foliage turns light green. Leaves can become narrow and distorted. (resembles Nitrogen deficiency)
Mo used in essential component of Nitrogen assimilation→ Nitrogen reductase enzyme
Toxicities occur at high pH (unusual but availability increases 100x for each pH unit increase) → reddish leaf margins
Symptoms: phosphorous deficiency, source/fertilizer, etc
Small leaves that may take on a reddish-purple tint. Leaf tips can look burnt and older leaves become almost black. Reduced fruit or seed production.
Fertilizer: Triple Superphosphate, bone-meal
Very dependent on pH range, optimum about pH=6.5.
Symptoms: Magnesium deficiency, source/fertilizer, etc
Slow growth and leaves turn pale yellow, sometimes just on the outer edges. New growth may be yellow with dark spots
Fertilizer: Epson Salts, other magnesium containing compounds
Symptoms: potassium deficiency, source/fertilizer, etc
Older leaves may look scorched around the edges and/or wilted. Interveinal chlorosis develops
Fertilizer: Compounds containing the words 'potassium' or 'potash'
Symptoms: chloride deficiency, toxicity
Yellowing and reduced leaf size
Toxicity: leaf tips and margin burn, death of roots tips
If pH decreases 1 unit, the plant availability of Zn2+, Cu2+, and Mn2+ ____
If pH decreases 1 unit: Zn2+, Cu2+, and Mn2+ ↑ increases 100x
If pH decreases 1 unit, the plant availability of Fe3+ ______
If pH decreases 1 unit: Fe3+ ↑ increases 1000x
If pH decreases 1 unit, the plant availability of Mo ______
If pH decreases 1 unit: Mo ↓ decreases 100x
If pH decreases 1 unit, the plant availability of B _______
B Remains Constant
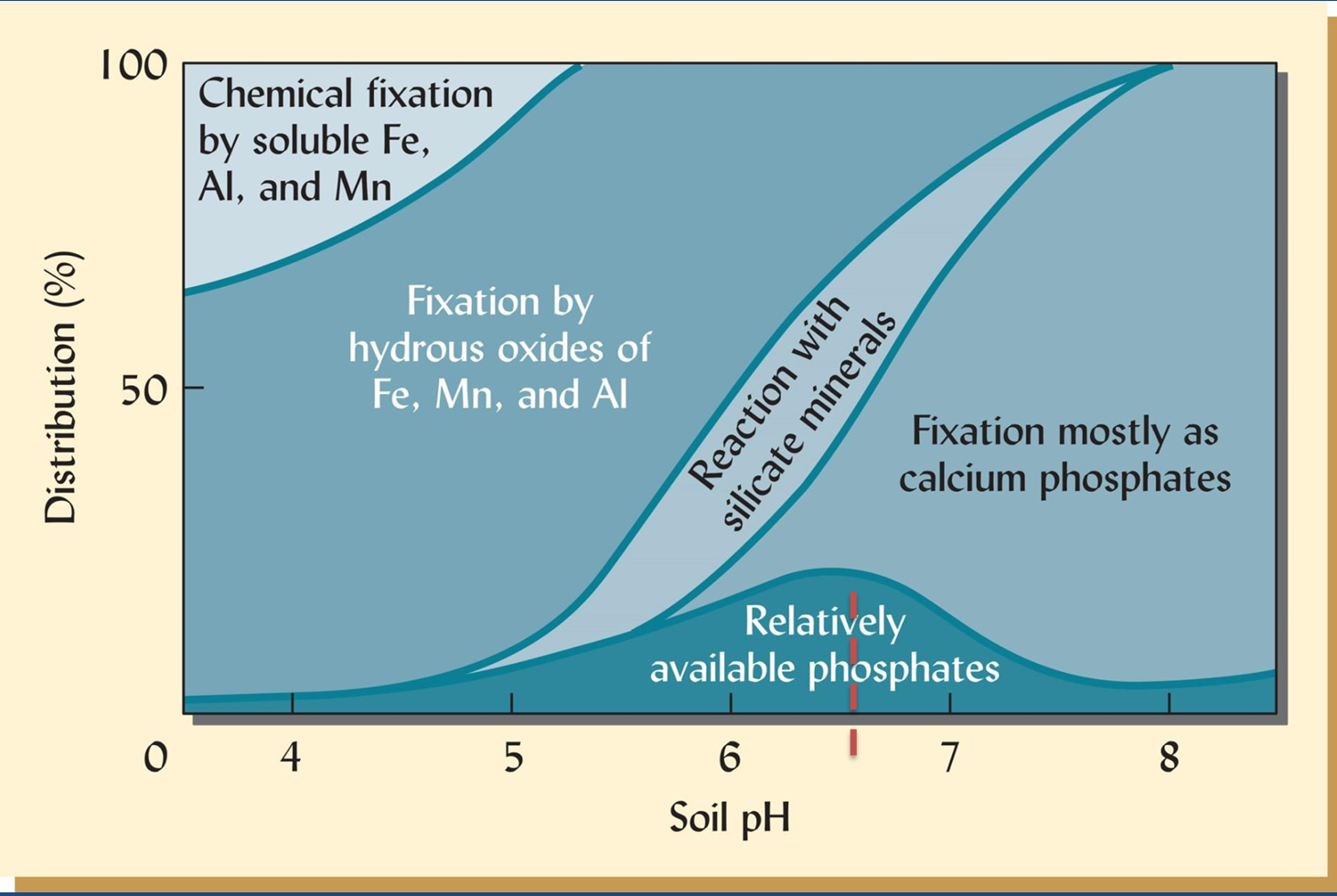
Multiple sources of mineral in soil? P example
The mineral with the lowest availability at a particular pH is the preferred (more stable) form.
So in this case, the Fe-P is the dominant form up to about pH=6, then above that pH, the Ca-P form will dominate.
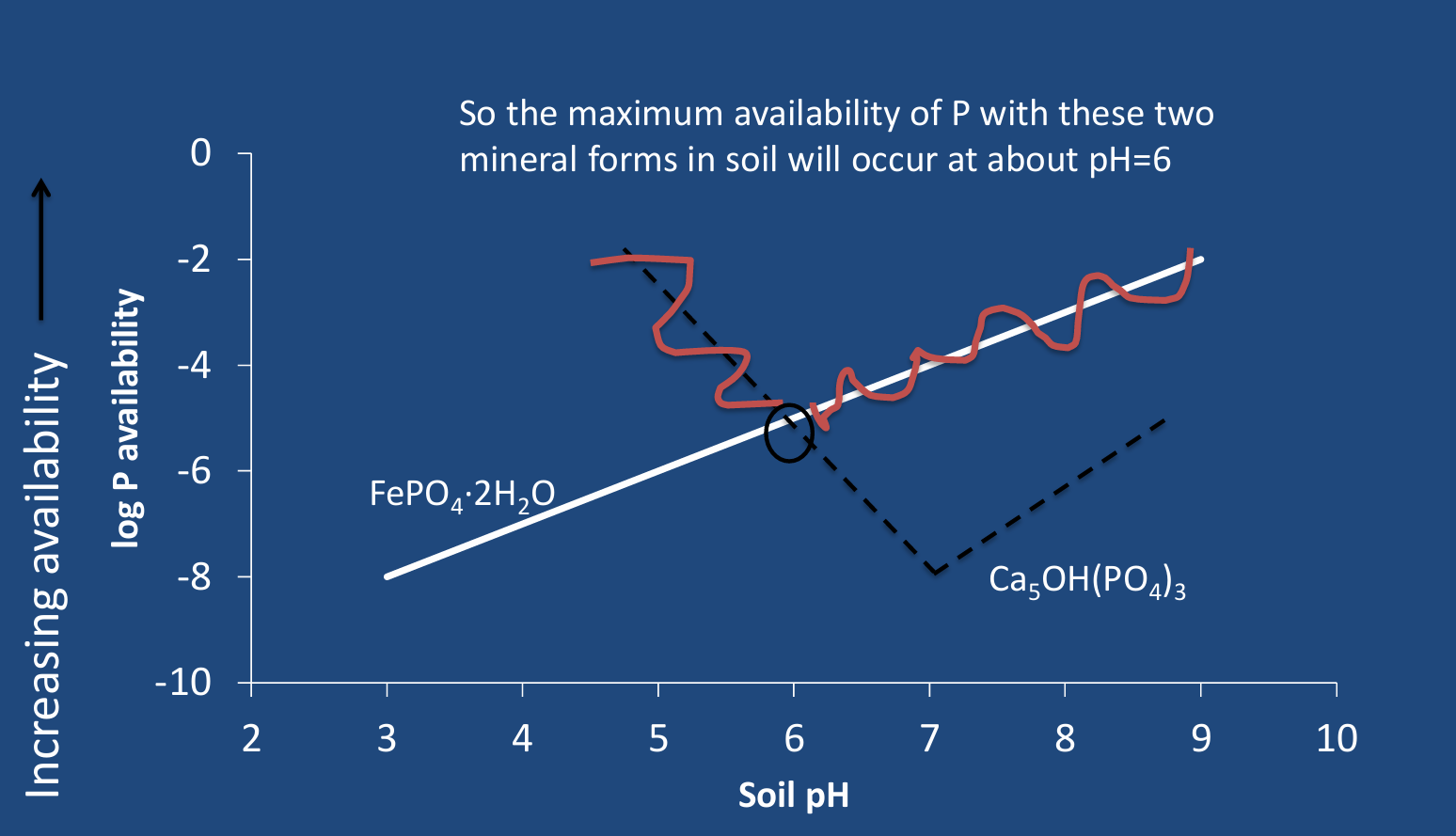
The P availability to plants is limited by fixation to a variety of mineral forms. The maximum availability is typically near pH=_____?.
maximum availability is typically near pH=6.
Six basic strategies that plant roots may use to uptake phosphorus from soils.
1) Increased root absorptive surface area.
2) Chelate iron or aluminum to release P.
3) Dissolve Ca–P compounds with acid exudates
4) Exude phosphatase enzymes to release P from organic compounds.
5) Exude substances to stimulate P solubilizing rhizobacteria.
6) Encourage colonization by mycorrhizal fungi that help plants take up P.
Describe use of chelators for iron uptake by plants:
Use of chelates to improve uptake of micronutrients, particularly metals
Nutrients that are otherwise strongly bound to soil solids can be made plant available by bringing them into solution with chelates
Chelators increase the solubility of Fe for plants:
When ferric (Fe3+) reduced to ferrous (Fe2+) at the root surface, iron releases and taken by the root.
Describe siderophores and the role they play in iron uptake by plants:
Siderophores : certain bacteria that produce highly effective iron chelating agents, roots release exudates to stimulate the growth of siderophores when deficient in iron, some. Siderophores bond with Fe3+ ions in the immediate vicinity of the root surface, greatly enhancing the amount of iron available for plant uptake
nitrogen losses (4)
-plant uptake and subsequent harvest
-nitrate leaching below the root-zone
- denitrification in wet soils
-ammonia volatilization in high pH soils or with NH3(gas) direct injection
About _____ as much P as N is required by plants.
About 1/5 as much P as N is required by plants.
C/P ratio
Mineralization of organic-P dominates at C/P <200:1
(net immobilization for C/P >300:1)
P cycle pools
organic, soluble, primary minerals, mineral surfaces (clay, oxides, carbonates)
*Notice that the plant removes P in solution which is in reaction with other pools
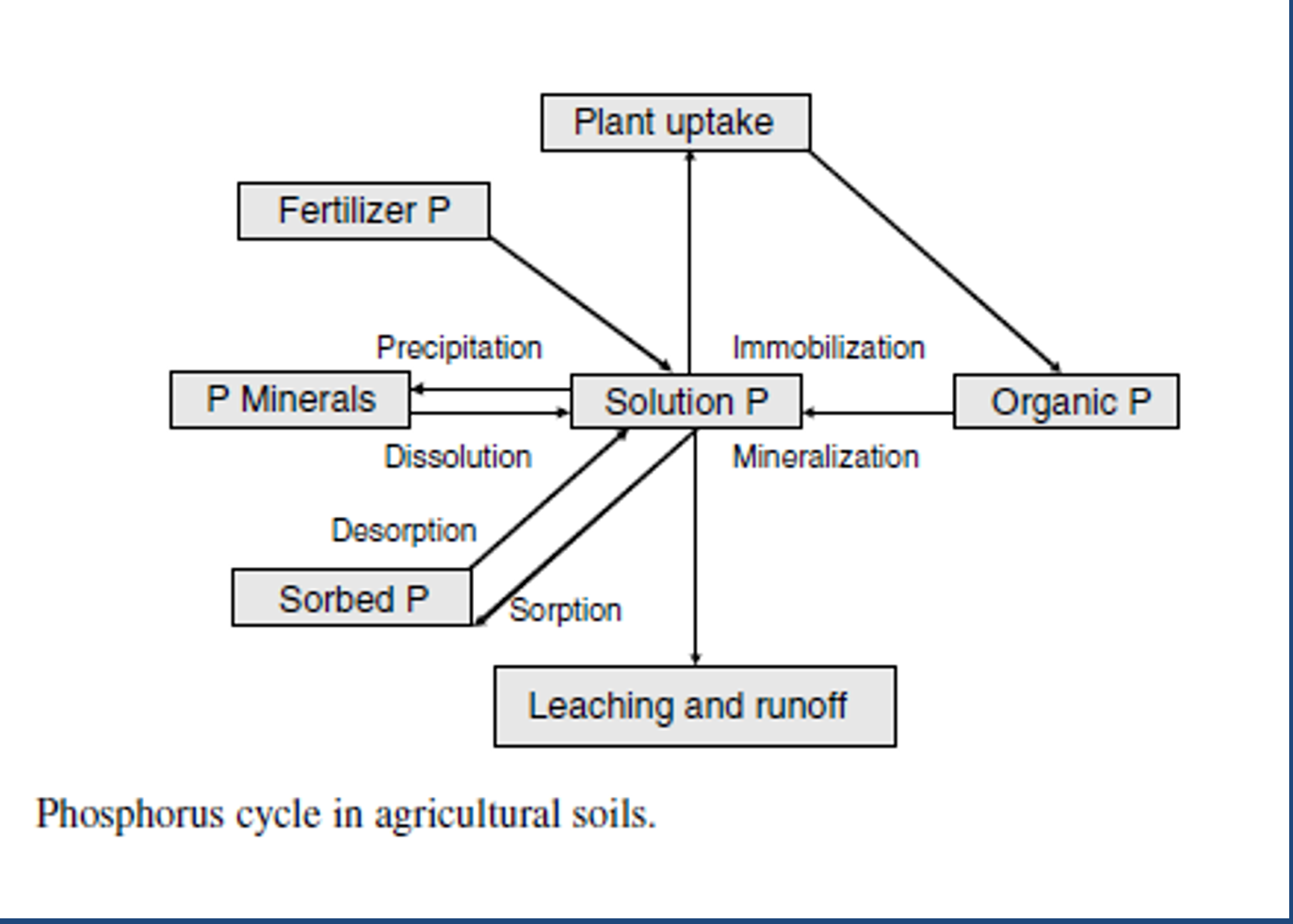
P cycle processes
immobilization and MINERALIZATION, WEATHERING, DESORPTION and adsorption to mineral surfaces, DISSOLUTION and precipitation into secondary compounds, plant uptake, leaching, runoff and erosion
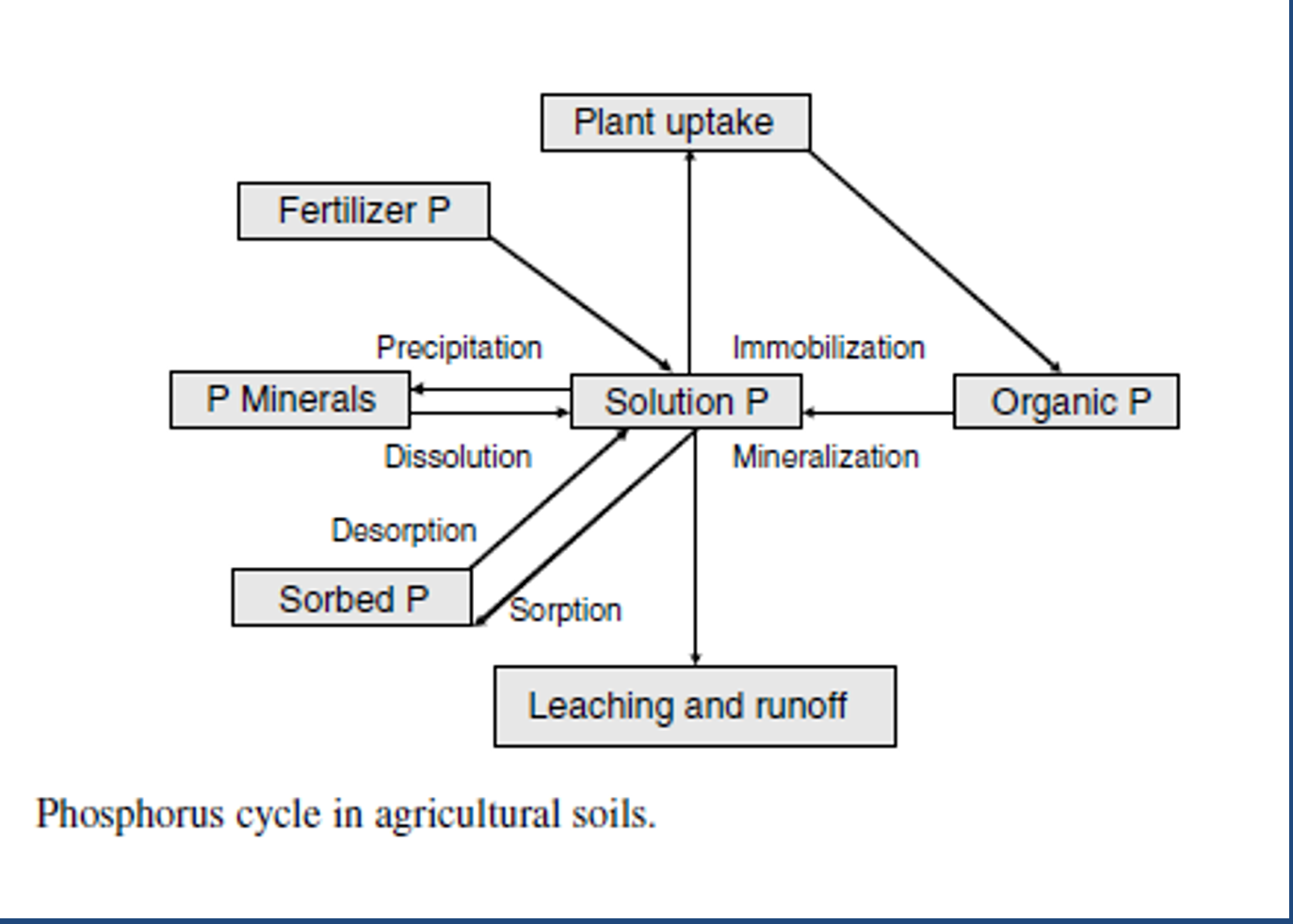
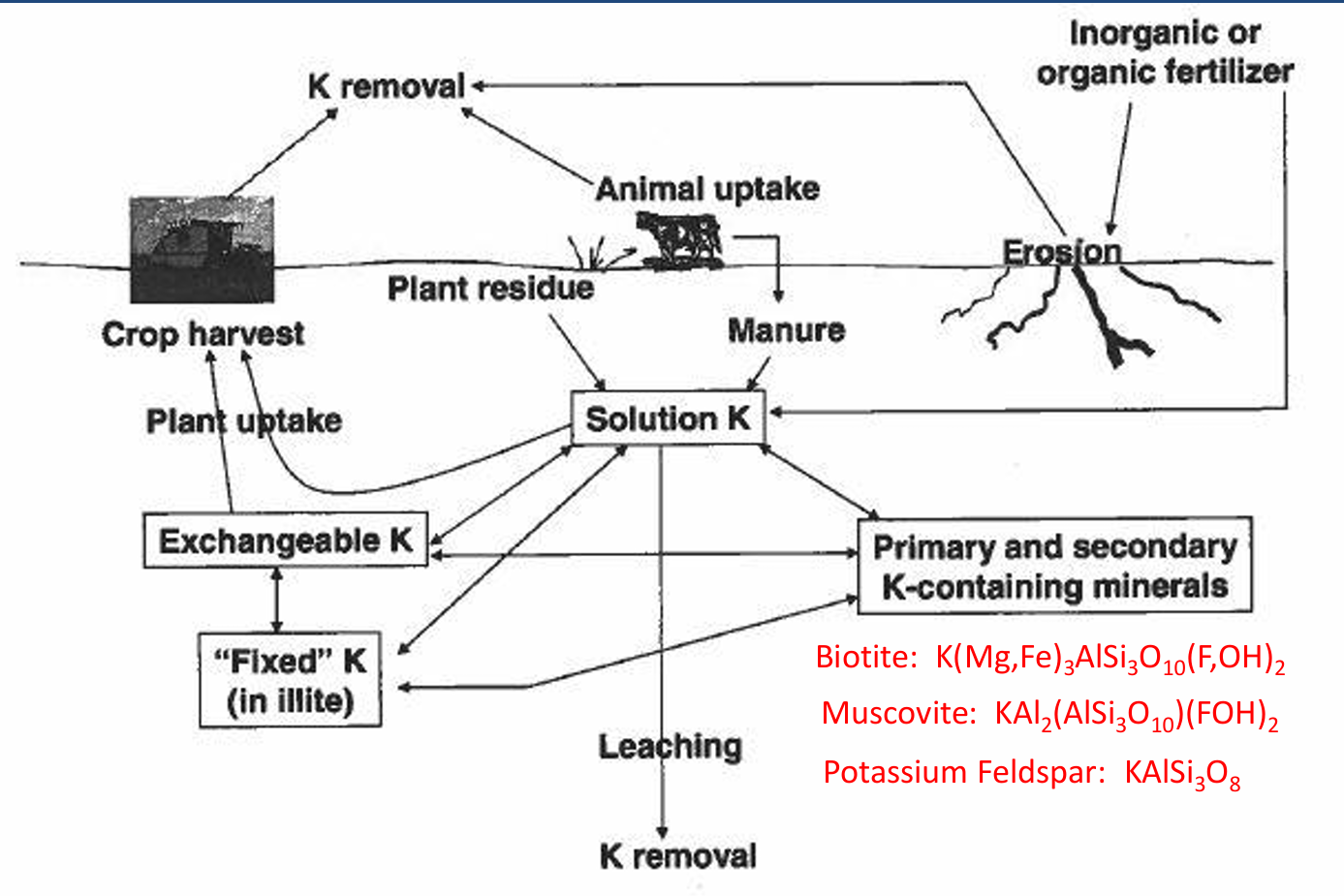
Most K in soils is in
minerals (mica, feldspars, etc.) and is unavailable.
soils in western US are rarely deficient.
K cycle pools
Exchangeable K, fixed K, soluble K, primary and secondary minerals
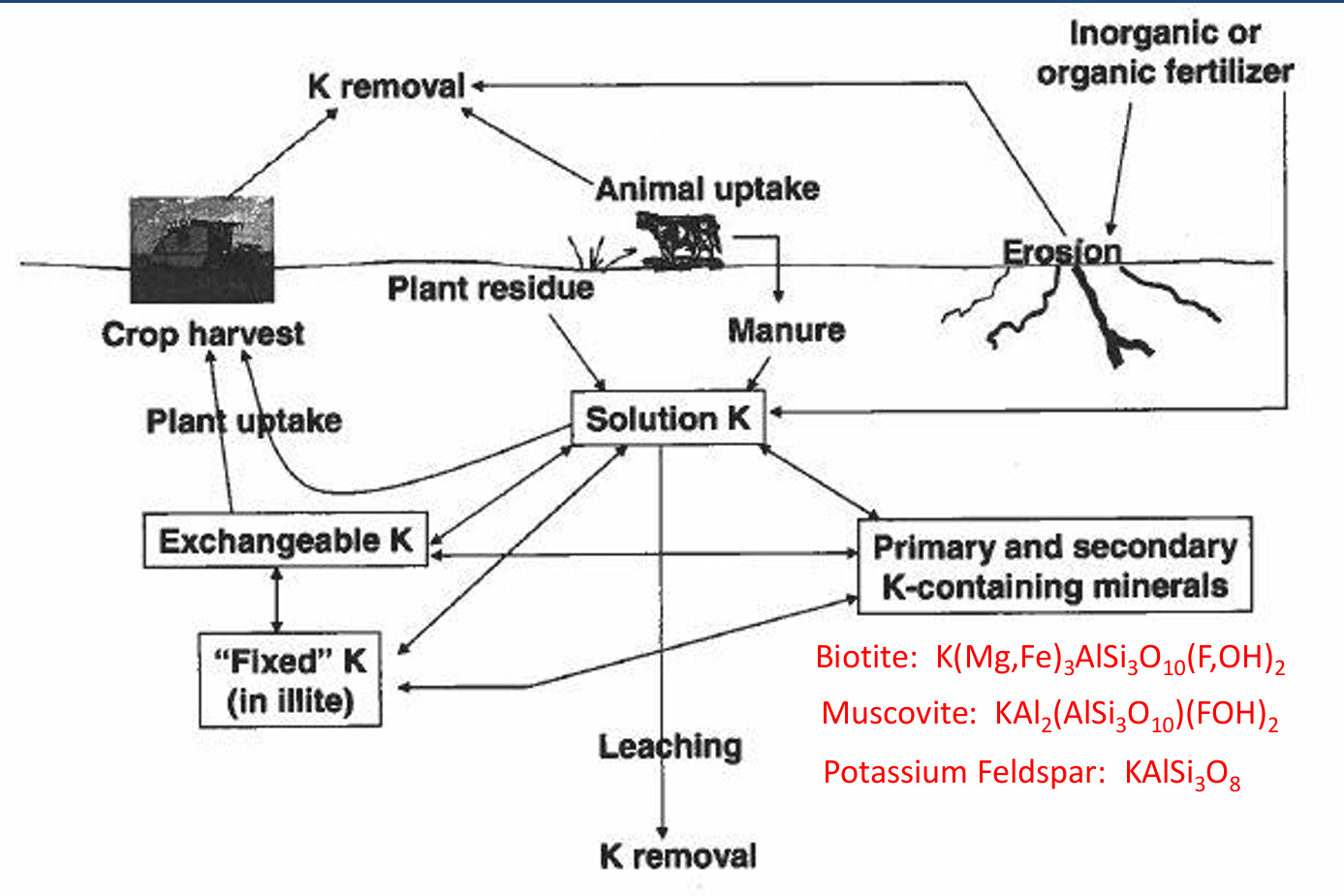
K cycle input
manure, plant residue, fertilizer
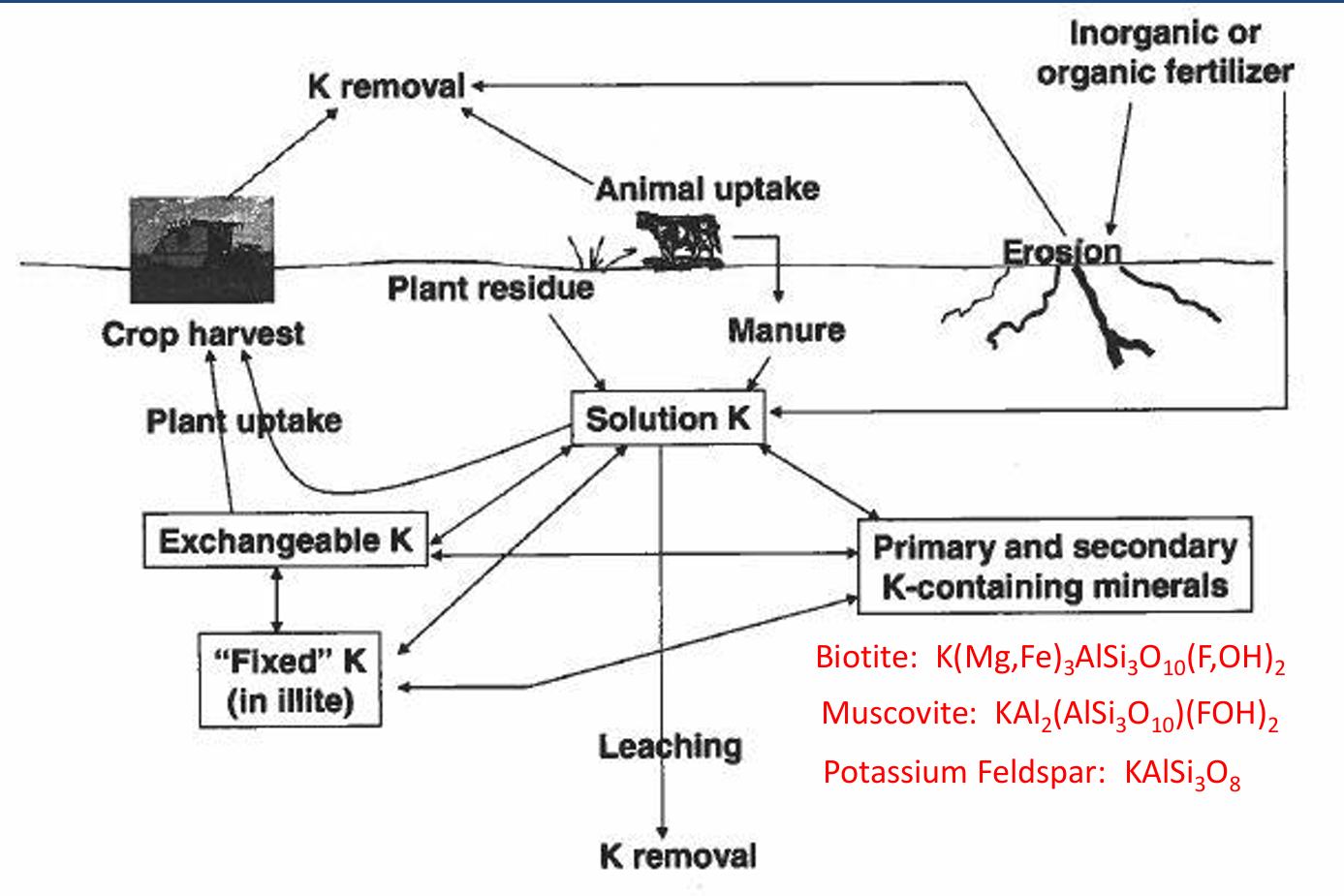
K cycle processes
animal uptake, erosion, plant uptake and crop harvest, leaching
most sulfur in soil source:
humus, gypsum as mineral source
S cycle pools
atmospheric sulfur, organic sulfur, adsorbed/mineral sulfur, sulfate, elemental sulfur, reduced sulfur
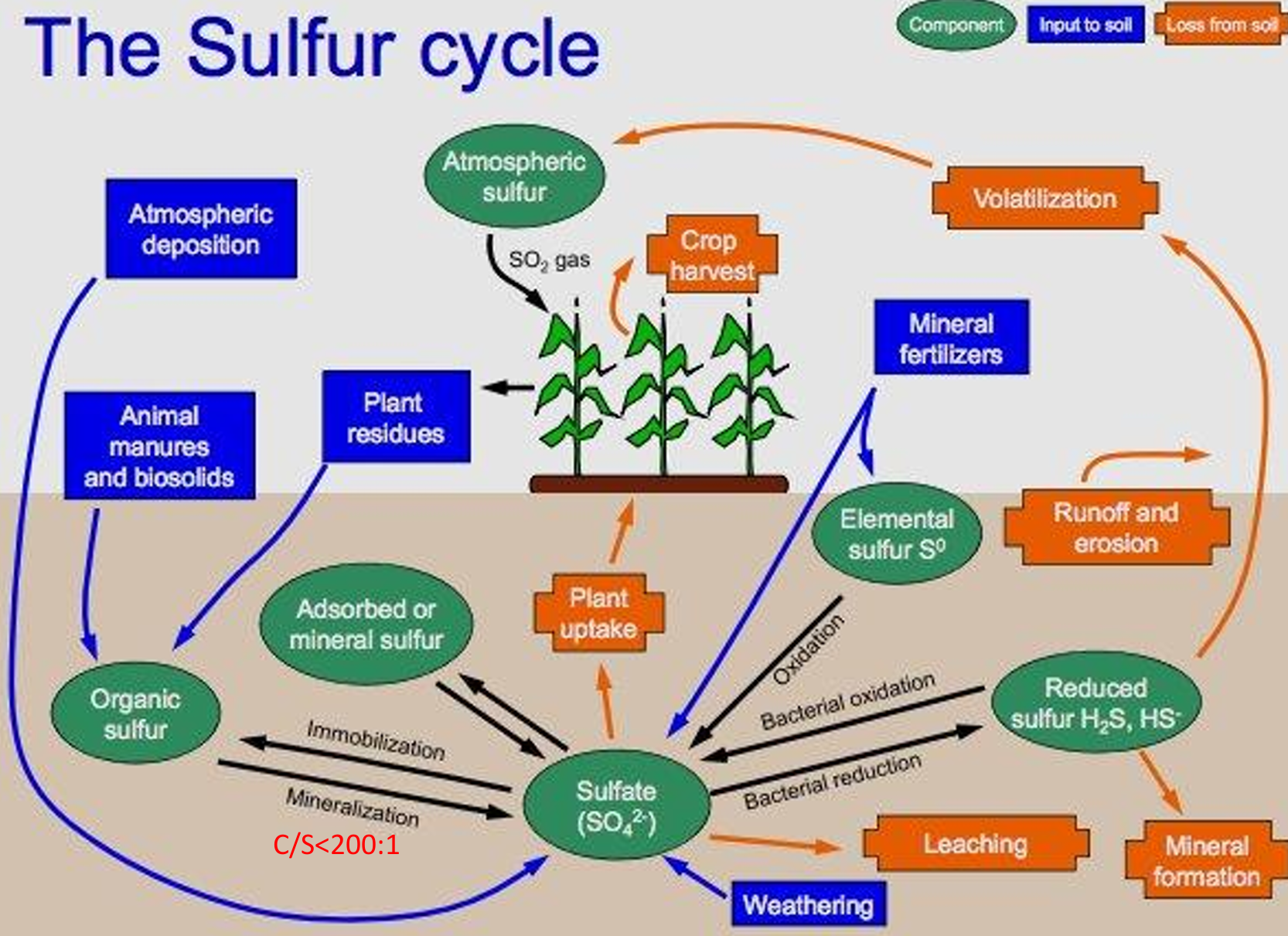
S cycle inputs
atmospheric deposition, manures, biosolids, plant residues, mineral fertilizers, WEATHERING
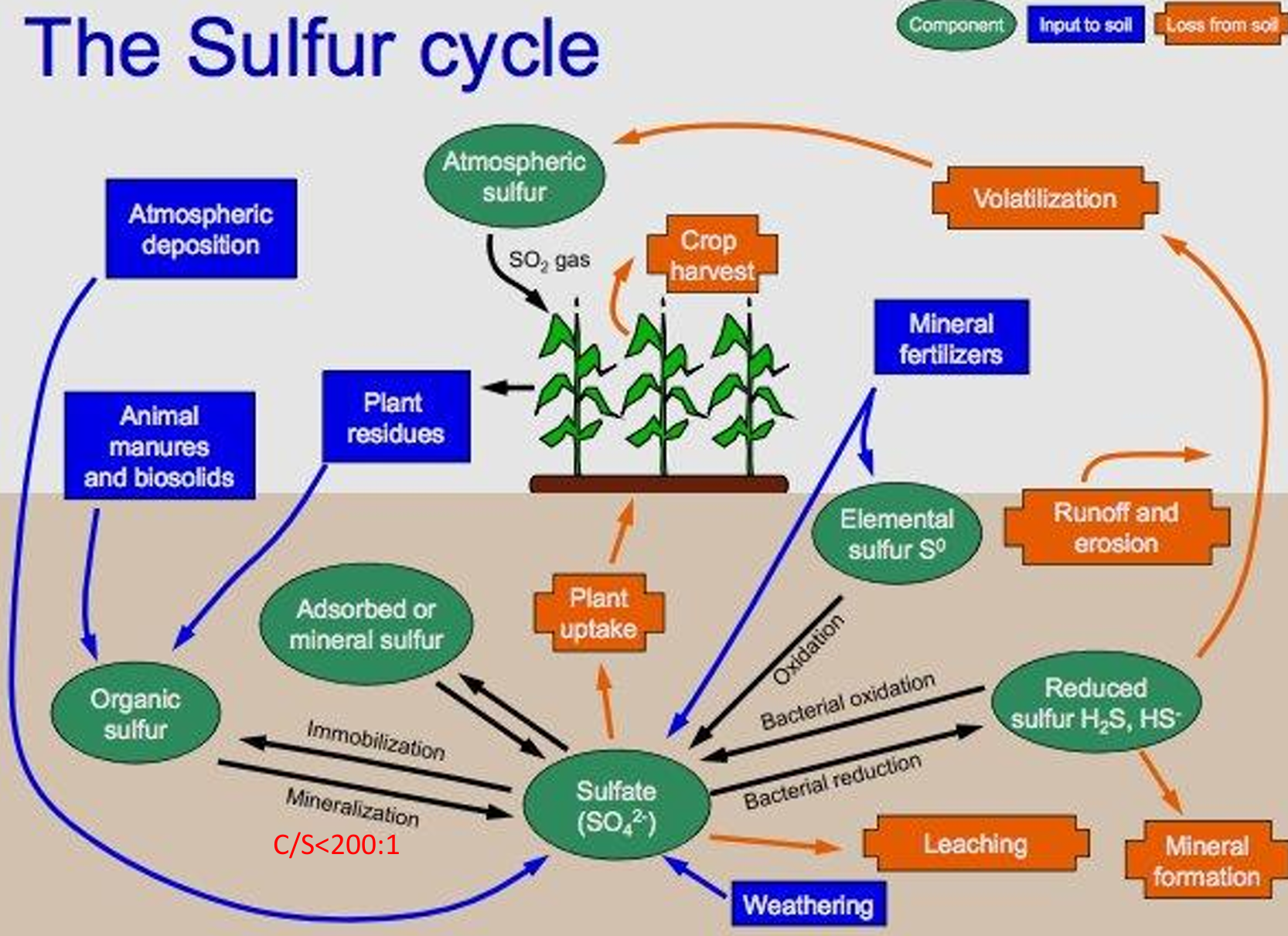
S cycle processes
immob. and MINERALIZATION, BACTERIAL OXIDATION and reduction, leaching, plant uptake, runoff and erosion, volatilization from reduced form
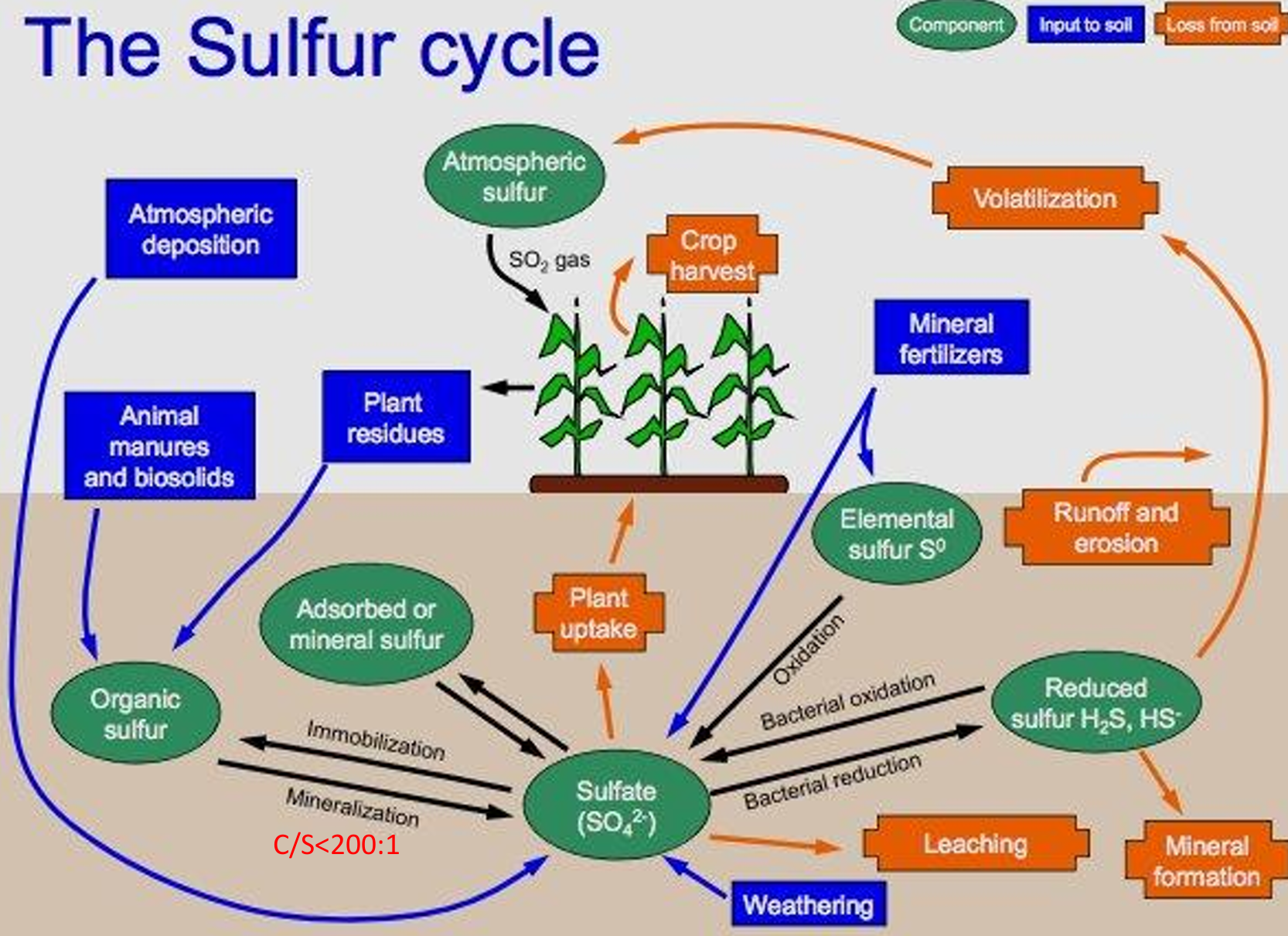
C/S ratio
Mineralization (organic-S → sulfate) for C/S <200/1,
immobilization for C/S>300/1.
Vegetables and legumes have ________ S requirements, grasses ________ S needs.
Vegetables and legumes have highest S requirements, grasses lowest S needs.
Sources for Calcium:
common, especially in arid and semi-arid soils. These include CaCO3 calcium carbonate or calcite), CaMg (CO3)2 (dolomite), and CaSO4·2H2O (gypsum).
calcium leaching
Ca is "held" in soil at cation exchange sites of clays and SOM, reducing its leaching and maintaining it in the root-zone for plant uptake.
In general, higher CEC soils are better reservoirs of plant nutrients and are typically more fertile than low CEC soils.
What happens to: P surplus in fertilized soils?
P goes to accumulation in soils and runoff losses
Phosphorus is less mobile than nitrate and more likely to be a runoff hazard when applied in excess and can eutrophy surface waters
Losses of P by leaching in drainage water are significant only at very high soil P levels
What happens to: N surplus in fertilized soils?
N goes to leaching and runoff, denitrification, and ammonia volatilization
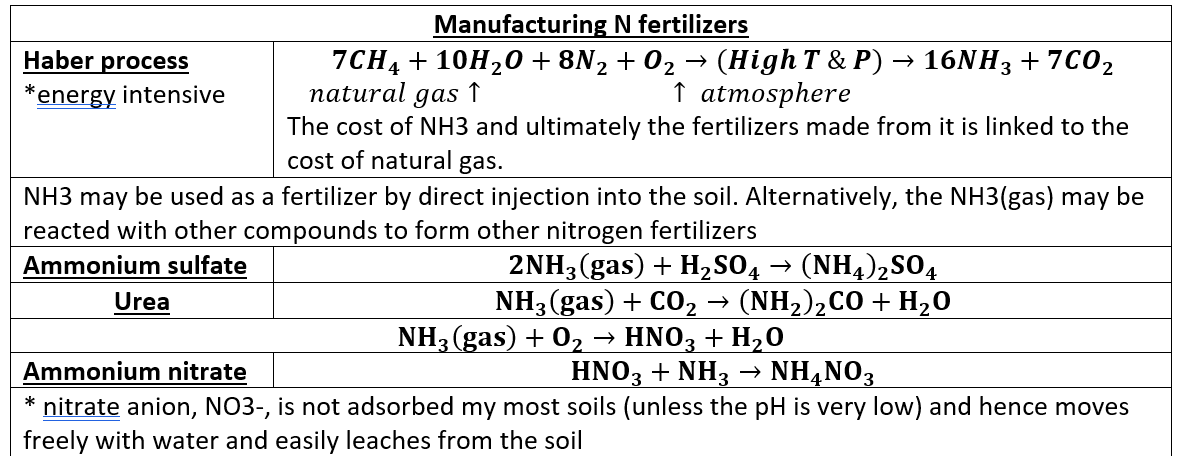
The nitrate anion, NO3-, is not adsorbed by most soils (unless the pH is very low) and hence moves freely with water and easily leaches from the soil.
Fertilizer fates
Not all the fertilizer added will be plant available because it can react with the soil solids, perhaps be taken in by microorganism (immobilization), and leach or runoff.
Nitrogen fertilizers are made through what process? and what is the chemical result?
manufactured by the Haber process → ammonia (NH3)
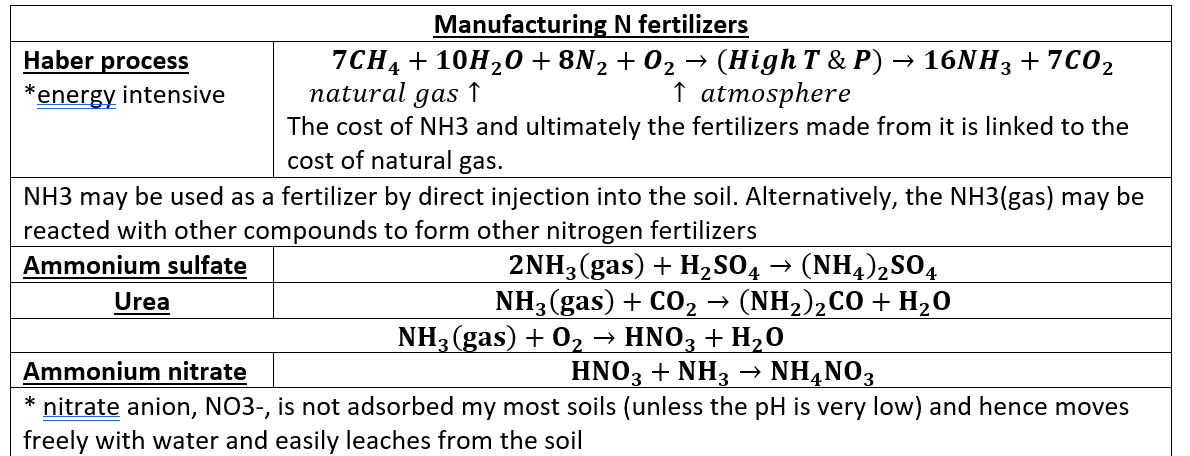
haber process
Natural gas + H2O + N2 + O2 → 16NH3 + CO2
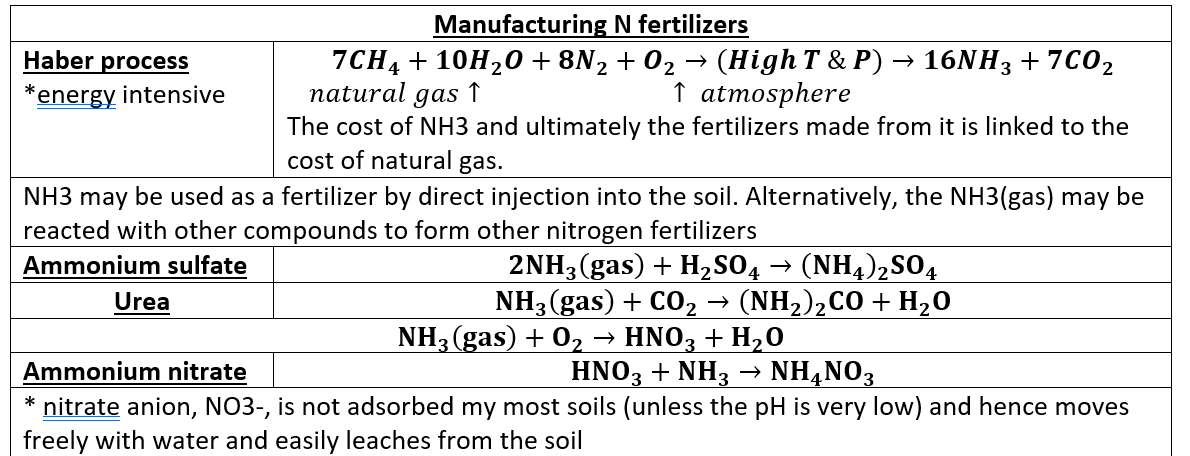
NH3 can be reacted with other compounds to make different fertilizers (ammonium sulfate, urea, ammonium nitrate)
common P fertilizers
Phosphoric acid
Triple superphosphate (a mixture of calcium phosphate and hydrofluoric acid);
Superphosphate (a mixture of calcium phosphate and gypsum);
Three types of fertilizer placement
Broadcast- Generalized, area wide application. Least efficient method but may be only practical method of application to established turf (lawns) or pastures.
Localized (targeted)- Fertilizer is placed in close proximity to plant (e.g. banding) to facilitate uptake and reduce loss to non-target areas/plants.
Foliar application or application through irrigation system (fertigation)
Note- the total fertilizer to be applied can be split into multiple applications of smaller doses to improve plant use efficiency.
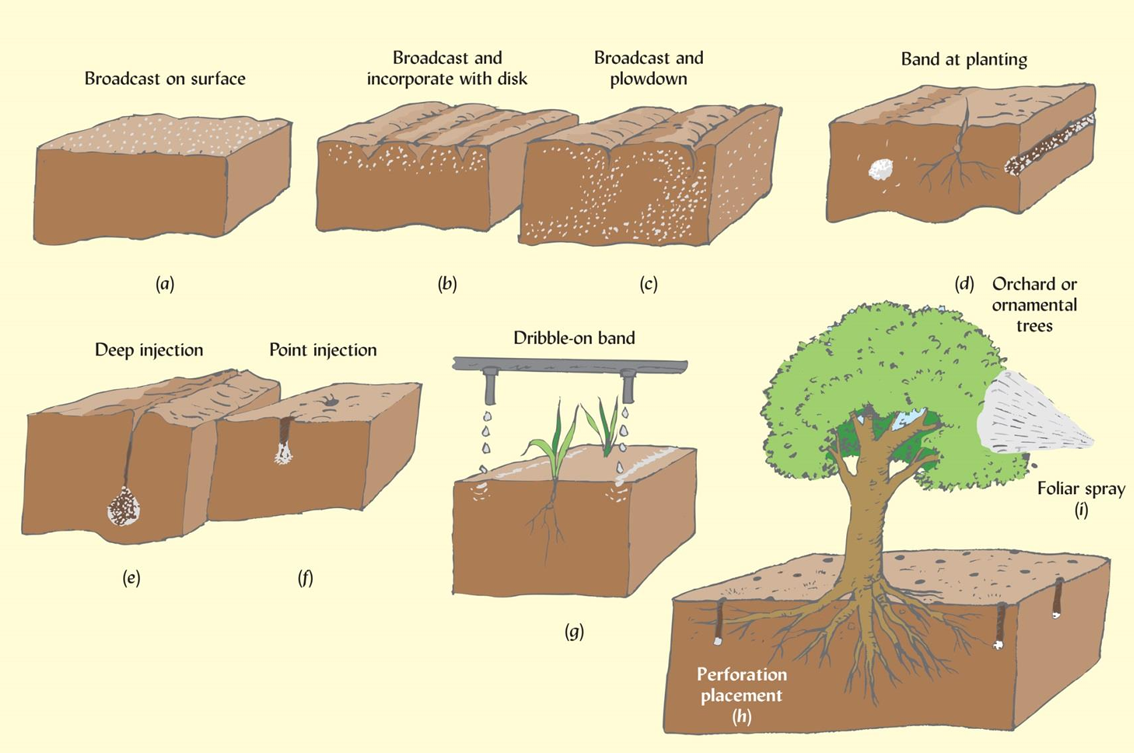
Alternatives to synthetic fertilizers:
Green manuring, Animal manure, Human manure (biosolids), Composted waste
Define: Green manuring
Incorporation of undecomposed green plant tissue, preferably legumes, into the soil.
issues with direct animal manure application:
Salts are a concern
Manure Spreading:
Manure spreading is very common, up to about 30% of the farmland in a count
nutrient content of different manure types:
Horse manure is at the low end of %N, sheep manure at the high end.
horse and llama are the least salty (lowest electrical conductivity)
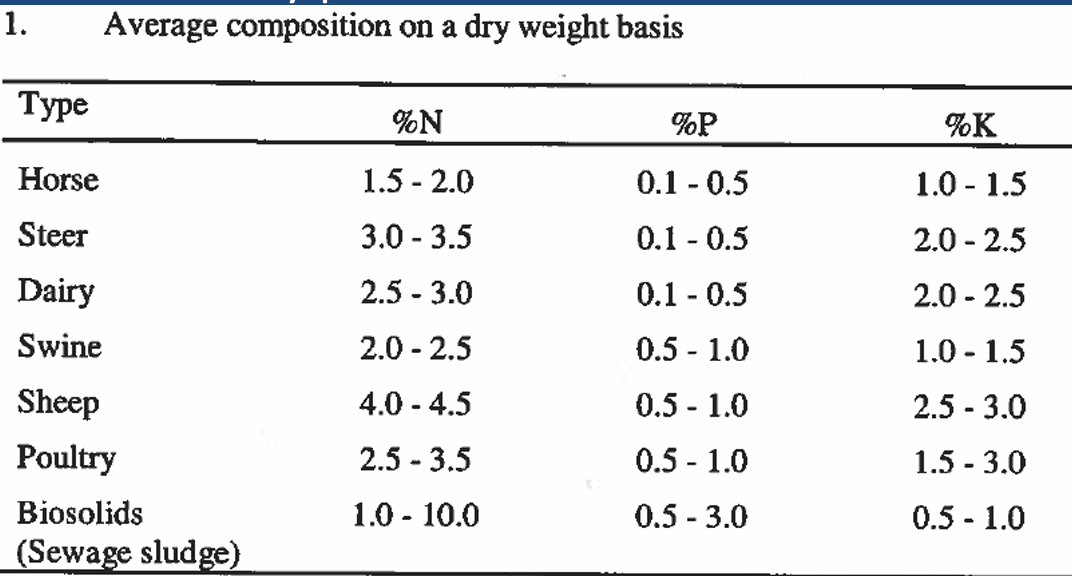
What must happen to organic forms of nutrients to become plant available?
Organic forms of nutrients must be mineralized before becoming plant available (slower release)
*Organic material stabilized by composting mineralizes more slowly than the raw material*
Define: Biosolids
organic materials resulting from the treatment of domestic sewage in a treatment facility
A concern with using biosolids:
metal content
Define: Composted Waste
Usually a combination of organic sources including manures, plant waste, and food waste.
Benefits of composting include:
destruction of pathogens and weed seeds (from heat), reduction of manure odor, creation of slower nutrient release fertilizer than raw manure
Organic material that is composted mineralizes ______ than raw/non-composted?
Organic material that is composted mineralizes slower than raw/non-composted
benefits of organic soil amendments (5)
Slower and prolonged release of N and P through mineralization. This reduces leaching loss, especially of N
Improved microbial habitat and associated mutualistic relationships with plants such as mycorrhizal fungi which improve the water and nutrient gathering of the plant.
Organic amendments increase the nutrient holding ability of the soil by increasing the CEC
Organic amendments improved soil structure that 1. stabilizes the soil against erosional losses (of solids and adsorbed nutrients) and, 2. improves aeration and water infiltration
Organic amendments increase the water holding capacity of soils and the plant available water
Micronutrient Toxicity symptoms: Fe, Mn
Fe: bronze/black leaf margins, dark simy roots, usually in submerged soils
Mn: Dark greenleaves with red flecks (early symptom), bronze,yellow interveinal tissue, may induce Fe deficiency
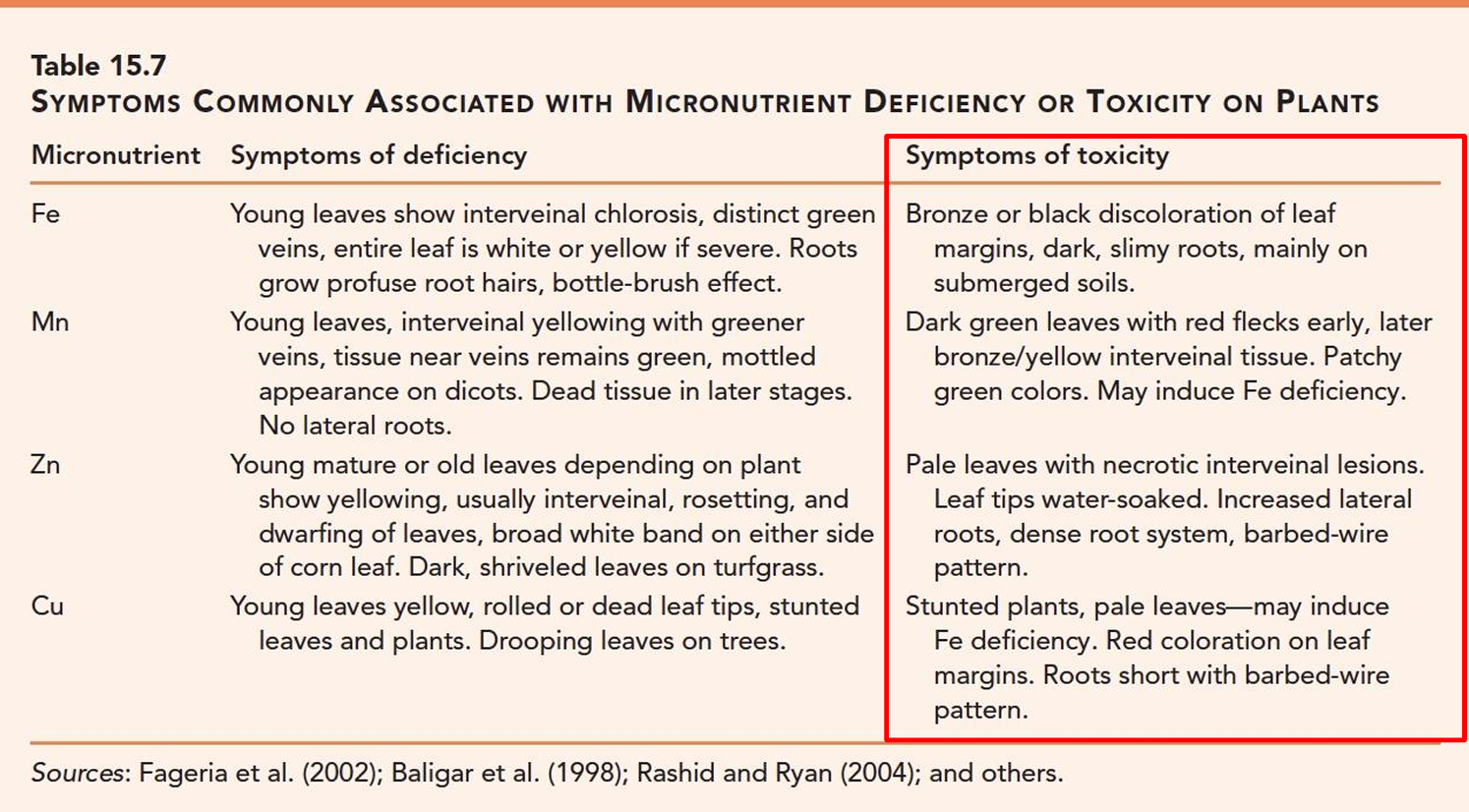
Micronutrient Toxicity symptoms: Zn, Cu
Zn: Pale leaves with necrotic interveinal lesions, leaf tips= water soaked. Increased lateral roots, dense roots
Cu: Stunted plants, pale leaves- may induce Fe deficiency, red leaf margins, short roots
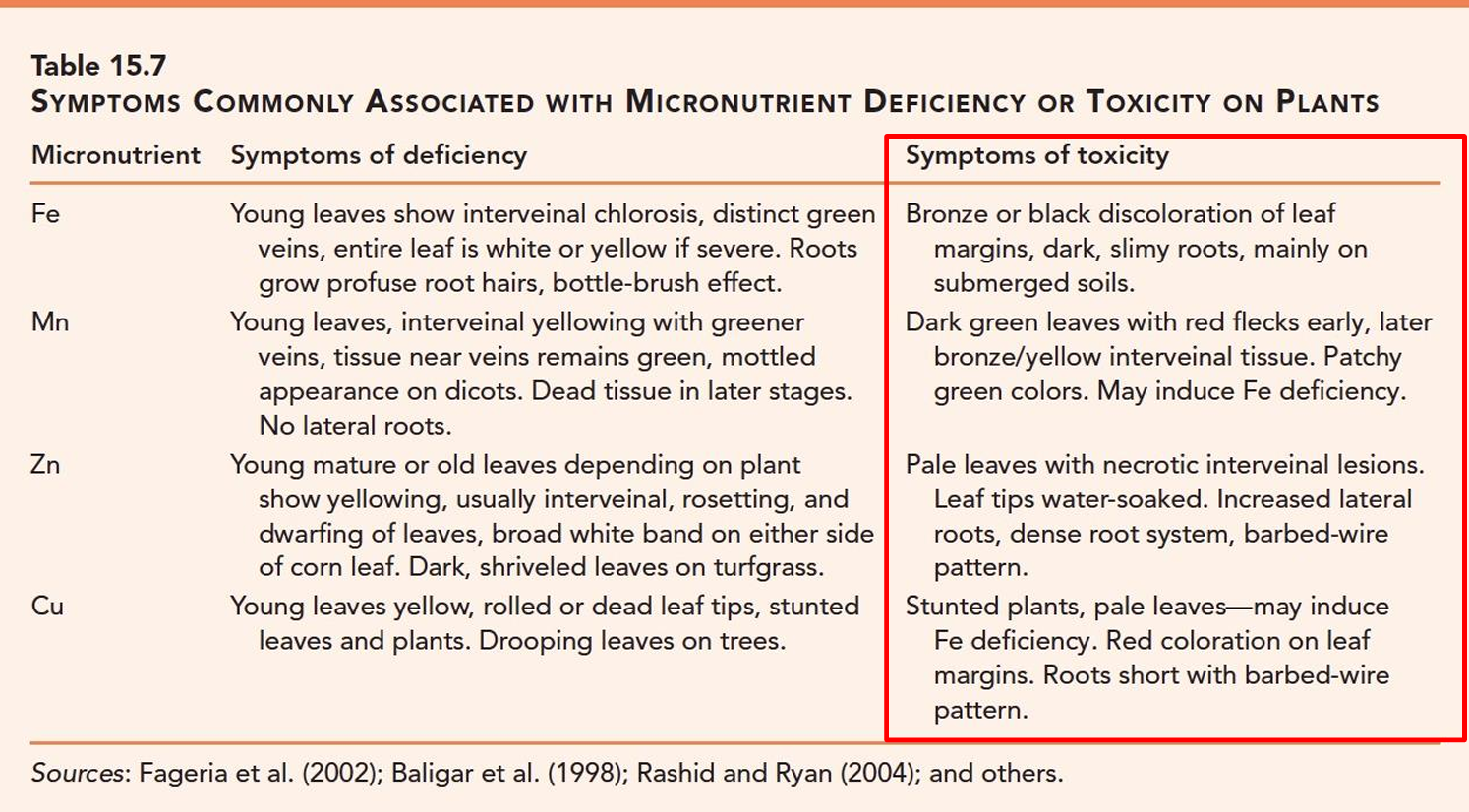
Micronutrient Toxicity symptoms: Ni, Mo
Ni: young leaflets distorted, white interveinal banding, dark green veins, streaking (white stripes/brown patches) brown stunted roots
Mo: Reddish leaf margins, normal roots