Electrolytes
1/253
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
254 Terms
What are body fluids composed of?
Water (solvent); minerals, electrolytes, vitamins, proteins, carbohydrates, lipids (solutes).
How are body fluids distributed?
Intracellular (WBCs, RBCs, tissues) and extracellular (extra- and intravascular).
How are body fluids distributed extravascularly?
Interstitial and transcellular fluid.
What are the functions of body fluids?
1. Dissolve and deliver substances to cells.
2. Blood volume (hyper- and hypotension).
3. Remove waste via urine.
4. Help maintain body temperature.
5. Protect and lubricate body substances.
How do body fluids protect and lubricate body substances?
1. CSF protects brain and spinal cord
2. Amniotic fluid protects fetus
3. Synovial fluid lubricates joints
4. Pleural fluid lubricates lung's surface
5. Pericardial fluid lubricates the sac in which the heart beats.
What are electrolytes?
Essential to all living matter, dissolve into ions, and can conduct electricity.
What are the functions of electrolytes?
1. Regulate osmotic pressure
2. Nerve signaling
3. Neuromuscular impulses
4. Acid-base balance
5. Enzyme cofactors
Which electrolytes regulate osmotic pressure?
Na+
Which electrolytes transmit nerve signals?
Na+ and K+
Which electrolytes conduct neuromuscular impulses?
Ca2+, Mg2+, K+
Which electrolytes regulate acid-base balance?
HCO3-, Cl- shift
Which electrolytes operate as enzyme cofactors?
Mg2+, Ca2+, K+, Fe2+, Zn2+, Cl-, Br-
What is the body's major extracellular cation?
Na+
What is the body's major intracellular cation?
K+
What is the body's major extracellular anion?
Cl-
What is the body's major intracellular anion?
(PO4)3-
What is the body's second-most abundant extracellular anion?
HCO3-
What is normal Na+ plasma concentration?
135-145 mM
What is normal K+ plasma concentration?
4 mM
What is normal intracellular K+ concentration?
141 mM
What is normal HCO3- plasma concentration?
28 mM
What is normal intracellular (PO4)3- concentration?
75 mg/dL
What factors influence electrolyte/fluid balance?
1. Ion transport
2. Hormones
3. Pressure
4. Lymphatic system
How is ion transport regulated?
1. Active transport of Na+ and K+ via Na-K-ATPase
2. Passive transport of Cl- and HCO3-
What can happen when the Na-K-ATPase malfunctions?
Serum K+ increases and Na+ decreases
What in-vivo etiologies explain Na-K-ATPase malfunction?
DM or hypoxia (energy- and/or O2-deficient cells).
What in-vitro etiologies explain Na-K-ATPase malfunction?
Chilling specimens inhibits the pump.
Which hormones affect electrolyte/fluid balance?
Aldosterone and Antidiuretic Hormone (ADH).
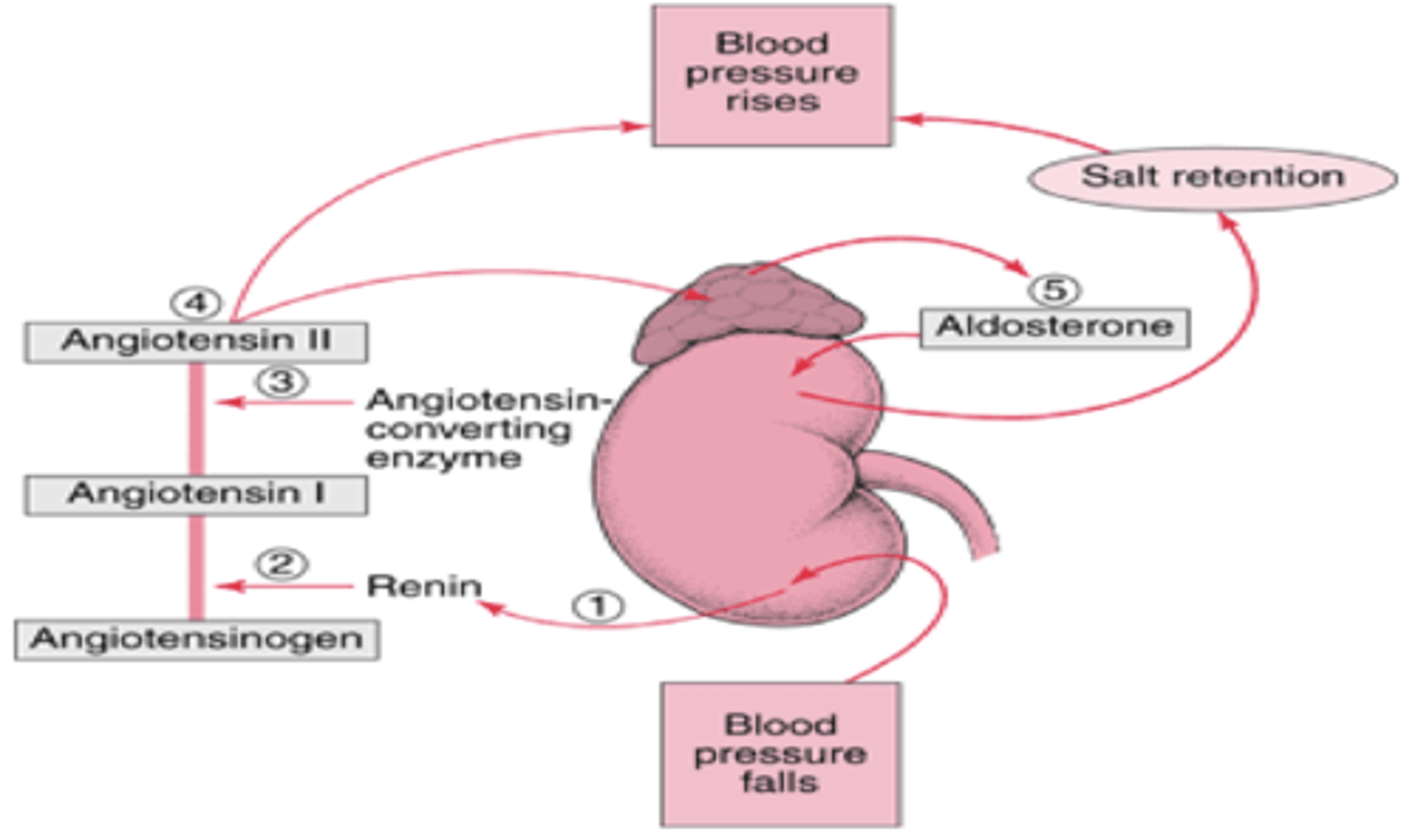
What stimulus triggers aldosterone release?
Apparent decrease in BV/BP (triggering RAS).
What is the site of aldosterone action?
Distal renal tubule and collecting duct.
What is the mode of action of aldosterone?
Stimulates Na+ (and thus water) reabsorption in exchange for K+ and H+ (Na+ SAVING).
Increased serum Na+ --> increased serum osmolarity --> increased BV/BP
What does hyposecretion of aldosterone cause?
Decreased BP/BV.
What does hypersecretion of aldosterone cause?
Increased BP and ECF expansion.
What is the effect of aldosterone on lab results?
Increased Na+, serum osmolarity, urine H+ and K+; decreased serum H+ and K+, urine Na+, urine osmolarity.
Fill in the blanks.

Answer.
Where is ADH produced and stored?
Produced in the hypothalamus, stored in the posterior pituitary.
When is ADH secreted?
When plasma osmolality increases but also when BV/BP decrease, regardless of osmolality.
What is the site of action of ADH?
Renal collecting duct.
What is the mode of action of ADH?
Increases the permeability of the collecting duct to water, increasing its reabsorption from the urine (H2O SAVING).
What problems are associated with ADH hyposecretion?
Diabetes Insipidus (DI), causing polydispsia/-uria. This leads to increased urine volume and serum Na+/osmolality and decreased urine osmolality/Na+.
What problems are associated with ADH hypersecretion?
Syndrome of Inappropriate Diuretic Hormone (SIADH), characterized by increased urine Na+/osmolality and decreased serum Na+/osmolality.
What is crystalloid osmotic pressure?
The osmotic pressure that depends on dissociated ions and small particles and regulates distribution of intra-/extracellular water to maintain cell shape.
How is crystalloid osmotic pressure expressed (units)?
As plasma (serum) osmolality, which total number of electolytes/solute (mol) per kg plasma - mOs/kg.
What is the plasma concentration and osmolality of Na+
135 mM and 270 mM (due to corresponding anion), respectively.
What is the plasma concentration of urea compared to the osmolality of urea?
14 mg/dL or 5 mM (does not dissociate into ions).
What is the concentration and osmolality of plasma glucose?
90 mg/dL or 5 mM (does not dissociate into ions).
What is the RR for serum osmolality?
280-310 mOs/kg (critical values < 265 or > 320).
What is isoosmolality?
Concentration of electrolytes is the same on either side of the cell membrane.
What is hypoosmolality?
Serum osmolality < 250 mOs/kg.
What could cause hypoosmolality, and what can it lead to if untreated?
Caused by SIADH (increased ADH) and/or hyperhydration. Can lead to cerebral edema.
What is hyperosmolality?
Serum osmolality > 320 mOs/kg.
What is hyperosmolality caused by, and what can it lead to if untreated?
Caused by DI, dehydration, and DM. Can lead to brain cell shrinkage.
How is measuring urine osmolality different from serum osmolality?
Urine osmolality RR is wide, as it significantly depends on water intake.
What does urine osmolality reflect?
Osmotically active constituents in the urine, such as Na+, K+, Cl-, urea, even vitamins, hormones, proteins, etc.
What does urine osmolality assess?
Body's state of hydration, the concentrating ability of the renal tubules, electrolyte balance.
Why are urine and serum osmolality useful together?
1. Differentiating DI and SIADH
2. Identifying renal tubule failure to concentrate urine.
How are urine/serum osmolality useful for differentiating DI and SIADH?
In DI, urine osmolality is low, while serum osmolality is high. In SIADH, serum osmolality is low, while urine osmolality > 200 mOs/kg (higher than normal).
How are urine/serum osmolality useful for identifying failure of the renal tubules?
Urine/serum osmolality is typically ~ 2-3. During renal tubule failure, it drops to 1.
How is serum osmolality estimated?
Osmo = 2 Na (mM) + (BUN/2.8) + (Glucose/18). Can be crudely approximated by 2 x Na.
What is the principle used when measuring osmolality?
Colligative properties, those dependent only on the number of particles in solution, not their identity. These include, boiling point elevation, freezing point depression, vapor pressure, and osmotic pressure.
What technique is used by the majority of labs to directly measure serum and urine osmolality?
Cryometer, which assesses freezing point depression of a sample. The higher the osmolality, the greater the freezing point depression.
What is osmolar gap?
Measured osmolality - estimated osmolality (RR: 0-10 mOs/kg).
What does increased osmolar gap indicate?
Excess amount of uncounted low MW particles present in plasma.
What endogenous substances could account for increased osmolar gap?
Ketone bodies (DKA).
What exogenous substances could account for increased osmolar gap?
Ethylene glycol, ethanol, methanol poisoning, making osmolar gap a good crude toxicology screen.
What is hydrostatic pressure? What does it do for the body?
Pressure exerted by a fluid in a system. Regulates distribution of water between intra-/extravascular spaces.
What is intravascular hydrostatic pressure? What does it do for the body?
Force of blood on the inner arterial walls/fluid pressure inside capillaries. Helps drive water out of the vessel into tissues and propels blood with heartbeat.
What is colloidal (oncotic) pressure? What does it do for the body?
Depends on large, colloidal particles (proteins, lipids). Regulates the distribution of water between intra-/extravascular spaces. The main regulator of BV/BP.
Which compound is the main regulator of colloidal/oncotic pressure?
Albumin.
What is edema?
Excess accumulation of interstitial fluid.
What are the common causes of edema?
Low serum protein, heart failure, or lymphatic blockage.
What dysfunction can cause low serum protein leading to edema?
Inadequate synthesis (liver disease), protein loss (renal disease), dietary deficiency.
How does heart failure cause edema?
High right-sided pressure cause an increase in capillary pressure, leading to increased fluid movement into the interstitium.
Which values are measured in a standard electrolyte panel?
Na+, K+, Cl-, HCO3-/CO2.
Which value is calculated in a standard electrolyte panel? How is this done?
Anion gap (AG or AGAP). Na+ - (Cl- + HCO3-).
Which electrolyte determines > 90% of serum osmolality?
Na+
What are the primary function of Na+ in the body?
1. Maintains electrolyte balance, and thus water distribution, between extra-/intracellular spaces, along with BP.
2. Required for nerve transmission (CNS) and muscle contraction
What factors regulate Na+ concentration in the body?
Na-K-ATPase, thirst, kidney function (aldosterone, ADH, renin).
Why is Na+ measured?
Assess hydration status and conditions involving electrolyte and/or water imbalance.
How must Na+ concentration be interpreted?
In the context of Na+/BV.
What is the most common electrolyte disorder, w/ diverse etiologies?
Hyponatremia.
What is the primary symptom of hyponatremia, and what is its cause?
Cerebral edema caused by hypotonicity.
How is hyponatremia categorized?
3 types based on measured serum osmolality:
1. Equal (isotonic)
2. Low (hypotonic)
3. High (hypertonic)
What are other names for isotonic hyponatremia? What is it characterized by?
Delusional hyponatremia, pseudohyponatremia. Characterized by normal water and Na+ levels.
What causes results of isotonic hyponatremia?
1. Using an indirect method to measure Na+.
2. Hyperlipidemia and hyperproteinemia.
What causes the error in indirect testing for patients with isotonic hyponatremia?
Indirect test assumes 93% water content. Since the patient has hyperlipidemia or hyperproteinemia, the water content is lower than what is assumed. Thus, volume assumed is larger than reality, causing lower concentration.
What are the two types of hypotonic hyponatremia?
1. Depletion (Na+ loss > water loss)
2. Dilution (water > Na+).
What are the two causes of depletion-based hypotonic hyponatremia?
1. Renal Na+ loss due to diuretics or decreased aldosterone production (Addison's Disease).
2. Extra-renal Na+ loss due to V/D, skin loss due to sweat (esp. when replaced with hypotonic fluid).
Which lab results help distinguish between depletion of Na+ from renal or extra-renal sources?
In renal depletion of Na+, urine Na+ is increased. In extra-renal depletion of Na+, urine Na+ is decreased.
What are the two causes of dilution-based hypotonic hyponatremia?
1. Euvolemic (normal Na+, increased H2O)
2. Hypervolemic cases (increase in Na+ < increase in H2O).
What are the main causes of euvolemic hypotonic hyponatremia?
SIADH, psychogenic polydipsia.
What are the main causes of hypervolemic hypotonic hyponatremia?
Edema-associated diseases (CHF, cirrhosis, nephrotic syndrome).
What is the main cause of hypertonic hyponatremia?
Dilution (increased H2O, normal Na+).
What is the primary cause of dilution in hypertonic hyponatremia?
High concentration of osmotically active moiety, such as mannitol infusion (exogenous) and/or uremia/hyperglycemia (endogenous).
What is hypernatremia?
Increased plasma Na+ concentration, a/w increased plasma osmolality, which can result in increased BV/BP.
What is hypernatremia caused by?
Osmotic flow of water out of cells:
1. Fluid deficit due to dehydration and water loss (renal or extra-renal).
2. Excess total body Na+ (ECF expansion) due to IV hypertonic saline use (primary cause) and/or increased renal conservation of Na+ (hyperaldosteronism).
What are renal and extra-renal causes of hypernatremia due to water loss?
Renal: DI, DM (osmotic diuresis), diuretics.
Extra-renal: V/D, sweating, burns.
What are the main functions of K+ in the body?
1. Regulation of neuromuscular excitability.
2. Contraction of heart muscle.
Why is K+ measured?
Identify cause/monitor treatment of hyperkalemia.