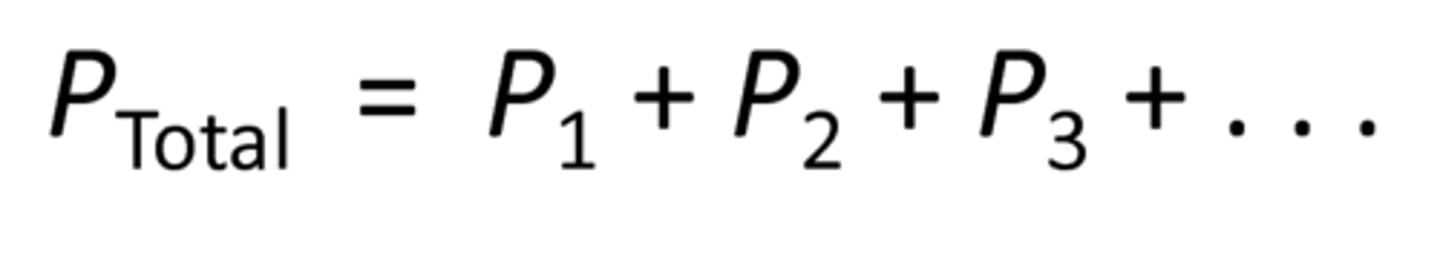Honors Chemistry Midterm Key Terms and Concepts
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
90 Terms
cm to inches
2.54 cm = 1 in
giga (G)
G 10^9
mega (M)
M 10^6
kilo- (k)
k 10^3
hecto- (h)
10^2
deka- (da)
10
deci- (d)
0.1
centi (c)
10^-2
milli- (m)
10^-3
micro- (μ)
10^-6
nano- (n)
10^-9
solid
Low energy
Defined shape
Defined volume
Ordered
liquid
Move freely
Takes shape of container
Defined volume
Medium energy
*** aqueous (dissolved in water)
gas
Undefined shape
Undefined volume
Always fills the container/space
High energy
Physical Properties
Substance fundamentally same
reversible
Examples:
Density
Melting
Mixing
Dissolving
Boiling
Chemical Properties
Having to do with a reaction
Substance has fundamentally changed
Irreversible
Examples:
Electrolysis
Rust
Fission
Burning
Density Equation
density = mass/volume
density of water
1 g/mL
less dense
floats
more dense
sinks
elements
Only one type of atom
Represented by an atom
Examples:
O2
N2
Cu
Na
compounds
A combination of different atoms/elements
Represented by a molecule
Examples:
CO2
H2O
NaCl
C6H12O6
ionic compounds
Give/takes an electron
Transfer electrons
Metal + Nonmetal
Conduct electricity in aqueous form
Examples:
NaCl
UF6
covalent compounds
Shares an electron
Nonmetal + Nonmetal
Examples:
CO2 HClO
CH4
H2O
HCl
NH3
organic compounds
ALL COVALENT BONDS
Natural, has to do with living things
CHONPS
Examples:
C6H12O6
CH4
mixtures
at least two substances
separate physically
Examples:
air
saltwater
pure substances
one substance
separate chemically
Examples:
O2
N2
CO2
H2O
heterogenous mixture
different composition
example: ocean water
homogenous
same distribution (uniform)
well mixed
example: air
solutions
homogeneous mixture in aqueous form
examples: milk, soda, alcohol
alloy
homogeneous mixture of metals
examples: bronze, brass, steel, sterling silver, white gold, rose gold
physical methods to separate homogeneous or heterogeneous mixtures
Distillation - separation based on boiling point
handpicking
magnetism - rone, nickel, cobalt
density - floating/sinking
filtration - separates solids and liquids
sieving - separates solids by size
evaporation
decanting - pouring off the top
isotopes
atoms with the same number of protons but different numbers of neutrons
atomic number
number of protons
mass number
number of protons + number of neutrons
cation
electrons are LOST, leaving a positively-charged ion
anion
electrons are gained, leaving a negatively-charged ion
average atomic mass
weighted average of the masses of an element's isotopes
two factors: percent abundance, mass number
alpha particle
helium atom
can be stopped by paper, skin, air
beta particle
electron
can be stopped by clothing, plastic
gamma ray
0/0 y - in the form of energy
can be stopped by lead, concrete
neutron
1/0 n
can be stopped by water, concrete, chemicals
proton
1/1 p or 1/1 H
half-life
time required for half of the original sample to decay
always the same within an element, but times vary dramatically
fusion
combining two lightweight nuclei to form something heavier
fission
splitting a heavy nucleus into two with smaller mass numbers
electromagnetic spectrum
gamma, x-rays, ultraviolet, infrared, microwaves, radiowaves (FM, shortwave, AM)
blue - high frequency/high energy
red - long wavelengths
Photon Energy (Planck Equation)
c=(lambda)(nu)
E = photon energy
h= Planck constant = 6.6261 x 10^-34 J*S
c = speed of light = 3 x 10^8 m/s
lambda = photon wavelength
nu = photon frequency
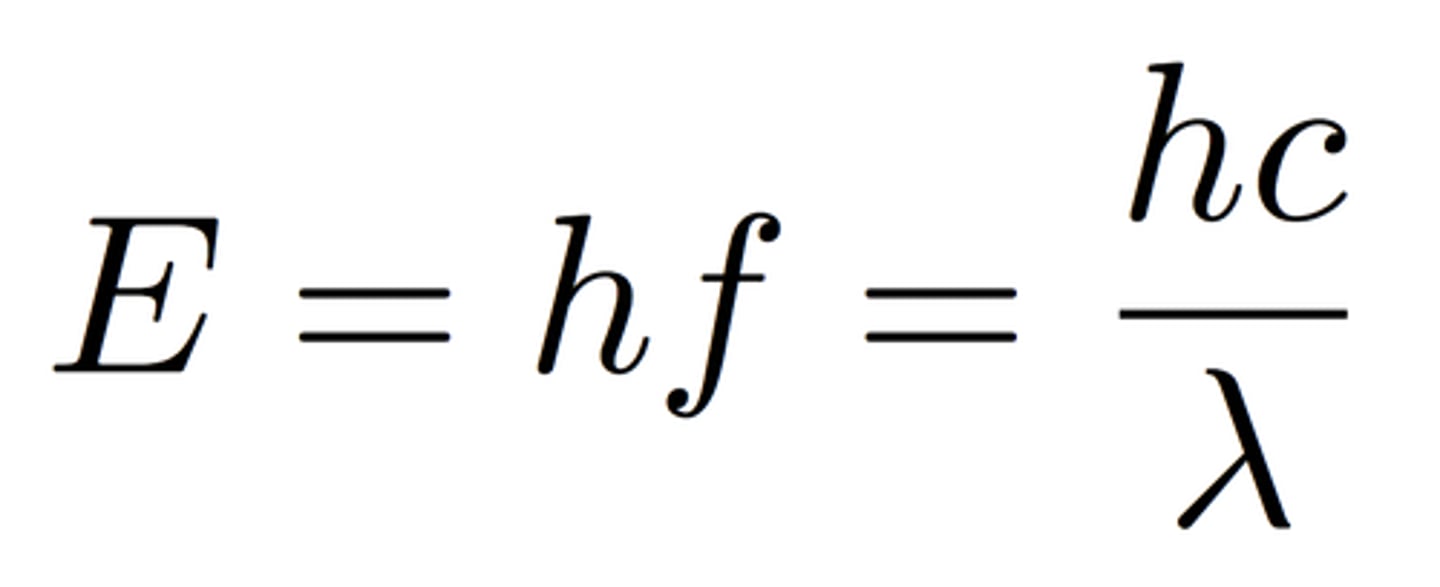
orbital labels
-The number tells the principal energy level.
-The letter tells the shape.
-The letter s means a spherical orbital.
-The letter p means a two-lobed orbital. The x, y, or z subscript on a p orbital label tells along which of the coordinate axes the two lobes lie.
orbitals different shapes
s, p, d, f
orbital filling rules
Aufbau Principle - fill from lowest energy to highest
N = 1 → 2 → 3 → etc.
s → p → d → f
Hund's Rule - orbitals fill with one electron before doubling up (think about a school bus)
Pauli Exclusion Principle - no two electrons can be identical, so the two that share an orbital have opposite spins
Democritus (400 BCE)
-all things are made of atoms, the fundamental invisible particles
-atoms can't be destroyed
-atoms are separated by the void, or empty space
-atoms come in different shapes and sizes, which explains their various properties
Dalton (1803)
-elements are composed of tiny particle called atoms
-atoms of a given element are identical
-atoms of different elements are different
-atoms of one element can combien with other elements to form compounds
-atoms are individsible in chemical processes, which involve the rearrangement of atoms
Thompson (1897)
Plum pudding model
-positively-charged sphere with negatively-charged electrons embedded
-stated that the atom was a single, uniform sphere of positive charge with negatively-charged electrons scattered throughout
Millikan (1909)
oil drop experiment
-proved that electrons had a fixed, negative charge that didn't vary
-discovered the charge on a single electron, known as the elementary charge (e)
Rutherford (1911)
Gold foil experiment
-showed that the positively-charged nucleus was localized over a very tiny volume of an atom, but took up most of its mass
-atoms are mostly made of empty space
-electrons orbited the nucleus in the center of an atom
Bohr (1913)
-electrons orbit the nucleus in specific paths or energy levels
-each shell has a specific max # of electrons it can hold
-tabeled using the principal quantam # (n) or the K,L,M,N,O,P,Q lettering system
-electrons can absorb energy to jump to higher levels or emit energy when returning to lower energy levels
Schrodinger (1926)
-describes electrons not as particles following fixed paths, but as waves existing in specific regions called orbitals
-model focuses on the probability of finding an electron in a specific region
- location of the electrons is referred to as an electron cloud
-varied densitives: a high density where an electron is most likely to be and a low density where an electron is least likely to be
Chadwick (1932)
-discovered the neutron, revealing that the atomic nucleus contains both positively-charged protons and neutral neutrons
-contributed to the discovery of nuclear fission and the development of the atomic bomb
-measured that the mass of a neutron was about the same as a proton
one mole
contains Avogadro's number of units
atomic mass on table is how many grams of that element are in one mole
Avogadro's number
6.022 * 10^23
writing formula from percentages
Assume 100 g (if necessary)
Determine how many moles of each element there are
Divide by the smallest number of moles to get whole number ratios
Multiple by 2 or 3 until every number is whole
molecular formulas
Compare empirical formula mass to the molar mass. They should be whole number multiples off.
Molecular formula = (empirical formula)n
Molar mass = n * empirical formula mass
diatomic elements
H N F O I Cl Br
charges of some metals
Ag - 1+
Zn - 2+
Cd - 2+
Ga - 3+
In - 3+
covalent compounds - prefix of second element
1 mono
2 di
3 tri
4 tetra
5 penta
6 hexa
7 hepta
8 octa
9 nona
10 deca
electronegativity
the ability of an atom to attract electrons when the atom is in a compound
ionization energy
The amount of energy required to remove an electron from an atom
atomic radius
one-half the distance between the nuclei of identical atoms that are bonded together
types of bonds
polar covalent, nonpolar covalent, ionic, metallic
dipole moment
Tail at positive end, arrow pointing toward negative end.
Higher electronegativity = partially negative
Lower electronegativity = partially positive
octet rule
all elements (except for H and He) want to reach 8 electrons in their outer shell. This is the most stable form. H and He are content with 2 (they're small)
lone pair
pair of electrons that aren't used in bonding
lewis structure exceptions
B and Be - smaller elements that often have less than 8 electrons
Larger noble gases and halogens - can have 'expanded octets' and hold more than 8 electrons. Often S, P, Xe, I, Cl
Atoms with odd numbers of electrons will have a single unpaired. This is called a free radical - often seen in N and O containing compounds.
types of intermolecular forces
1) London dispersion forces
2) Dipole-dipole interactions
3) Hydrogen bonds
4) Anything with an ion
Weakest : London - Dipole - Hydrogen : Strongest
intermolecular forces
forces of attraction between molecules
LDF
Electrons exist in a cloud
The cloud has a certain amount of randomness associated with it
At certain moments, there is a probability that all electrons are on the same side of the atom, creating an "instantaneous dipole"
Instantaneous dipole is a minor force of attraction
More electrons = more momentary dipoles = larger molecules
Dipole-Dipole
Polar molecules have dipoles
When multiple polar molecules are put together, the oppositely charged ends are attracted to each other
Stronger than an instantaneous dipole
More polar, less distance = stronger force of attraction
Hydrogen Bonding
Specific type of dipole-dipole
Extra strong because Hydrogen is tiny and happen when it's bound to something SUPER electronegative
Valid for H-F, H-N, and H-O
Water + H-Bonds
Capillary action
Ice crystalline structure
Glaciers, ice floating
Viscosity
Surface Tension
High specific heat
Effects of IMFs
Boiling point - stronger forces = higher BP
Melting point - stronger forces = higher MP
Vapor pressure - stronger forces = lower VP
Pressure of gas ABOVE the liquid
Ex: acetone (nail polish remover) has a really high vapor pressure
Viscosity - stronger forces = higher viscosity
Surface tension - stronger forces = stronger surface tension
Ideal Gas Law Assumptions
1) nonpolar
2) small
3) high temperature
4) low pressure
Kinetic Molecular Theory
1) continuous random motion
2) particles have no volume
3) pressure = collisions w/ wall
4) no attractions or repulsions
5) kinetic energy = temperature
Ideal Gas Law
PV = nRT
Boyle's Law
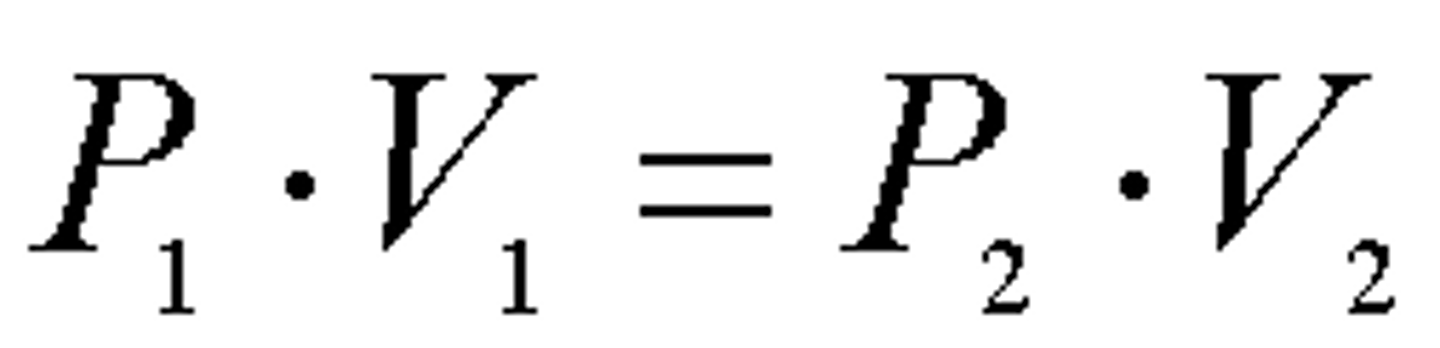
Charles' Law
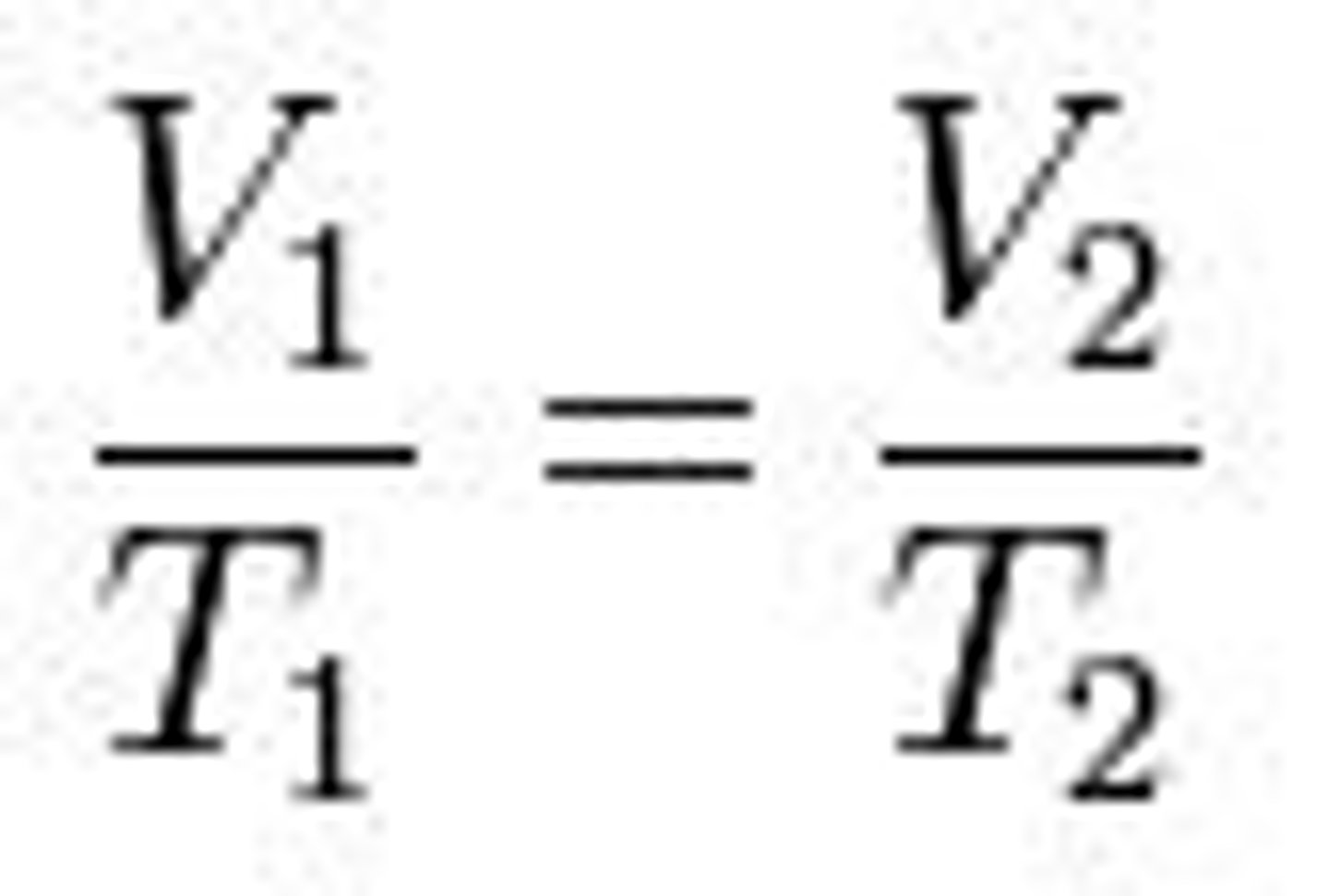
Avogadro's Law
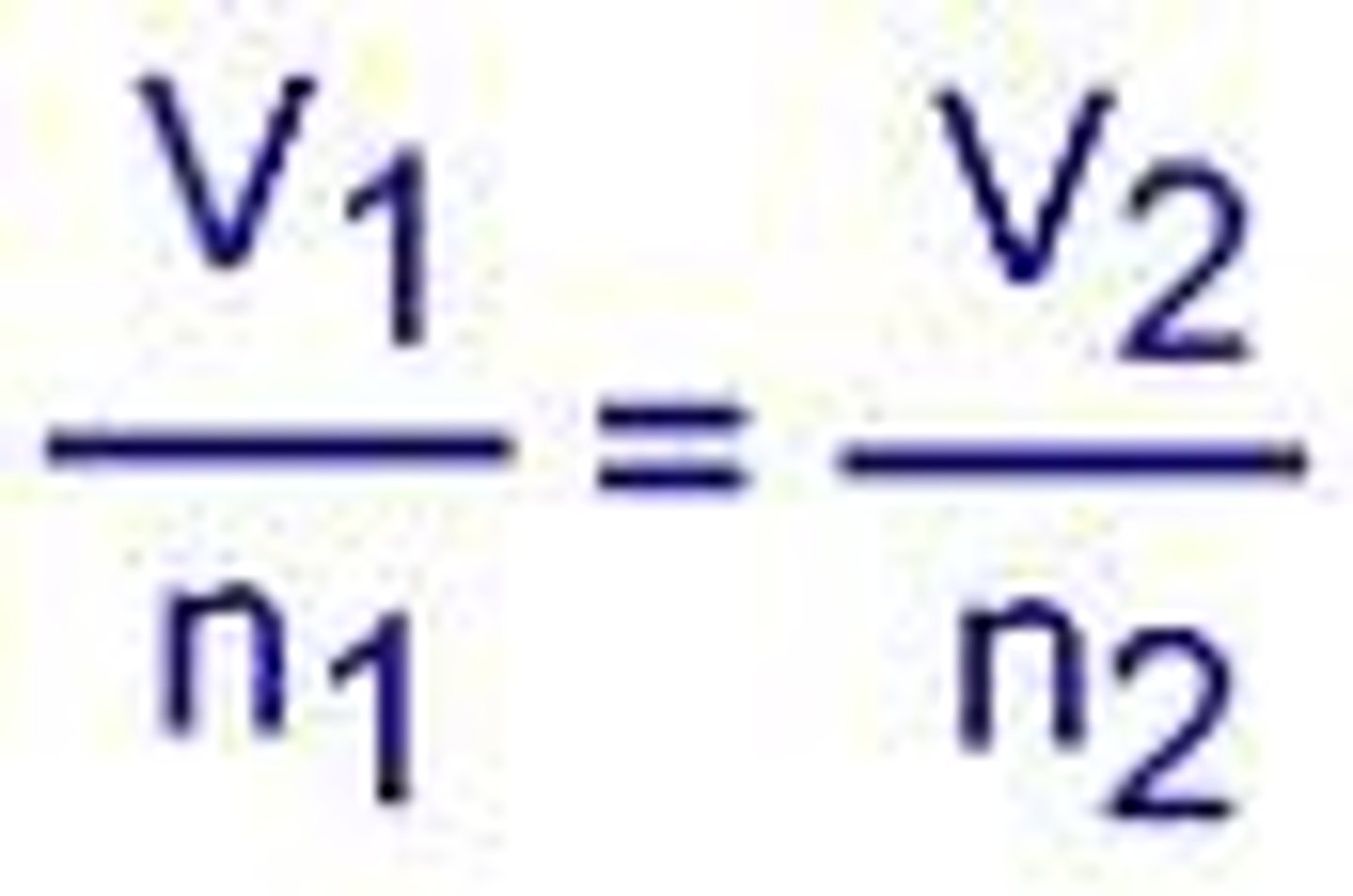
Amonton's Law
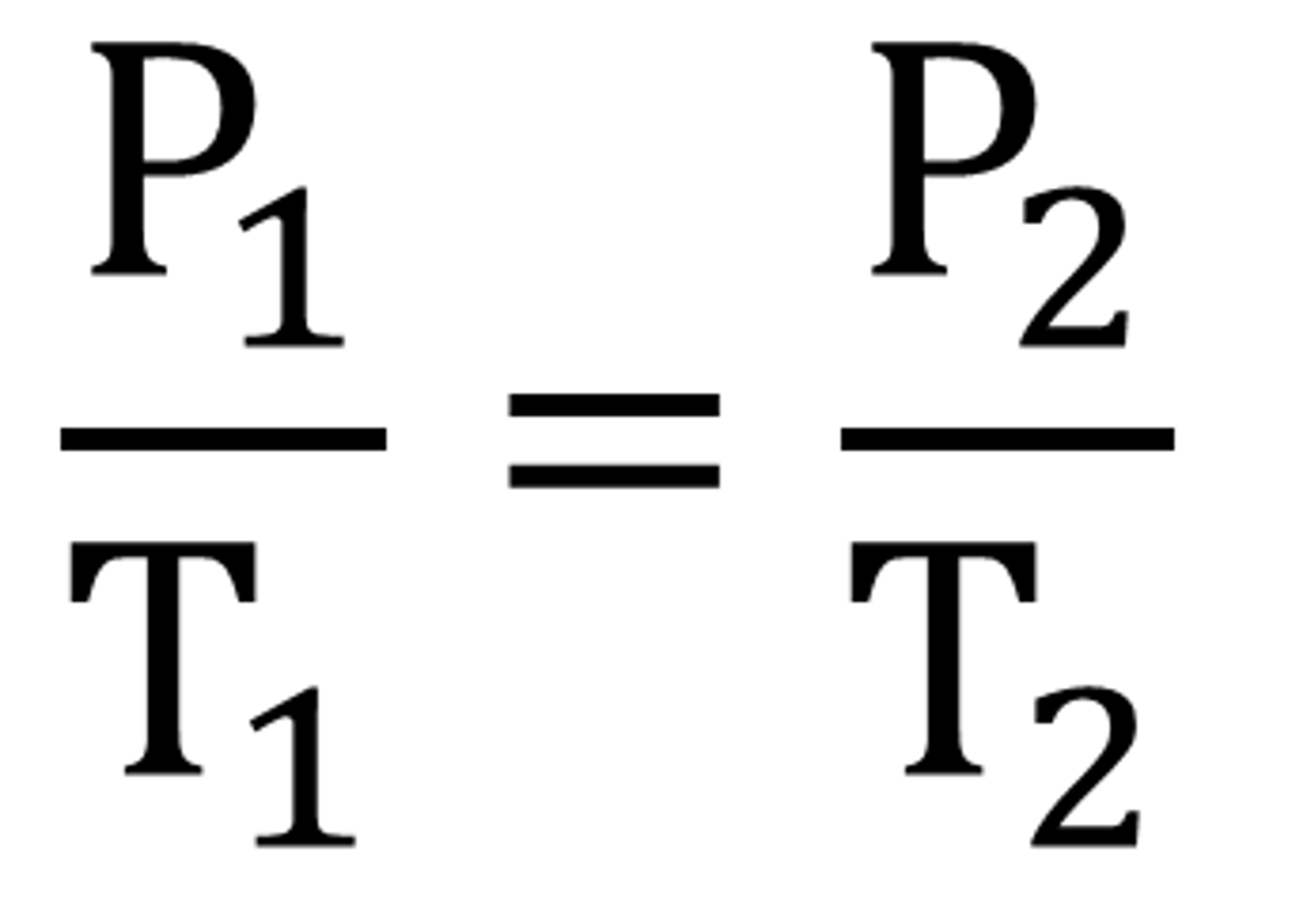
Combined Law

Dalton's Law of Partial Pressures