Theoretical Yield, Actual Yield, Percent Yield
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What does theoretical yield assume?
That every single last reactant molecule will react to form a product
What do we calculate with stoichiometry?
Theoretical yield
What does stoichiometry equal?
Theoretical yield
What is actual yield?
How much product we actually get in our reaction
What is actual yield always less than?
Theoretical yield
Why is actual yield always less than theoretical yield?
Because there are errors
What needs to be met for a reaction to take place?
Certain conditions
What are the three conditions that need to take place for a reaction to happen?
Molecules must collide, they must collide with enough energy to break bonds, and they must collide with correct orientation (need to be facing the right way)
What happens if not all three conditions are met?
No product
What is the percent yield?
Just like any other percent we have ever done
What is the formula for percent yield?
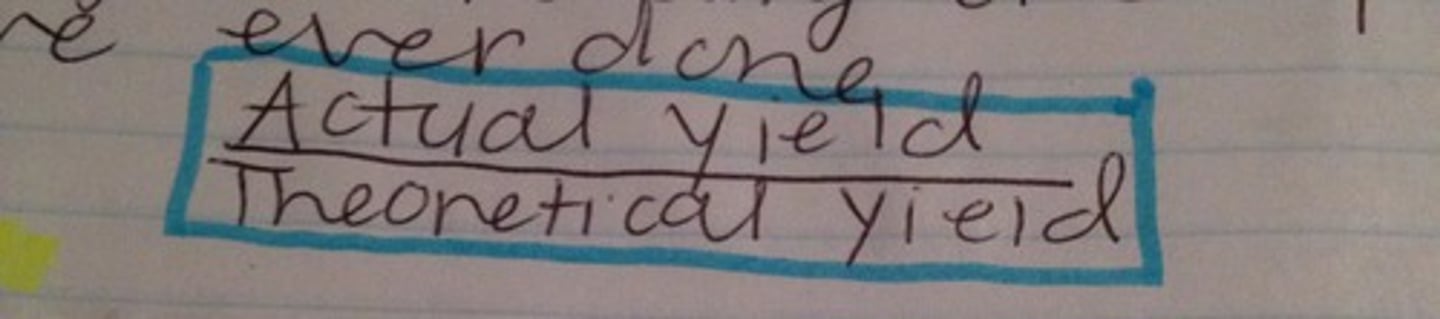
In the analogy identify the theoretical yield, actual yield, and percent yield. The test was marked out of 135 pts. You scored 83 pts. Your percentage was 61%.
Theoretical-135
Actual- 83
Percent- 61%
If 6.57 g of iron are reacted with an excess of hydrochloric acid, HCl, then hydrogen gas and 17.63 g of iron (III) chloride are obtained. Calculate the theoretical yield and percent yield of FeCl3.
Theoretical- 19.08 g FeCl3
Percent- 92.4%
What are the 5 steps for doing a yield problem?
1. balance the chemical equation
2. convert to moles
3. conversion factor
4. convert to desired units
5. find % yield by using formula