Aleks Chapter 10 | CHEM10401
1/54
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
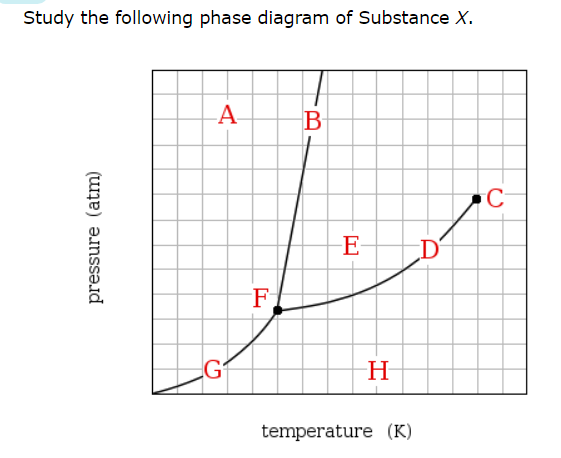
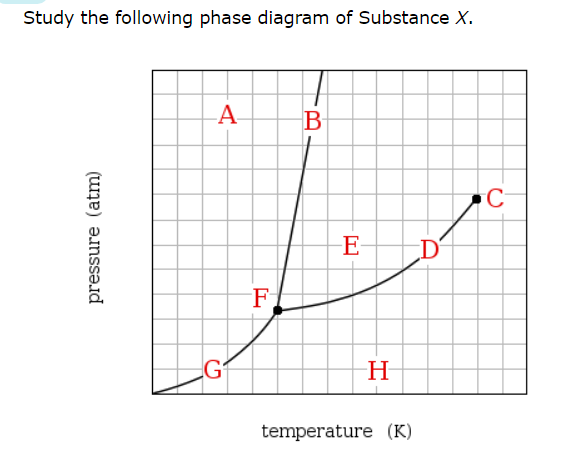
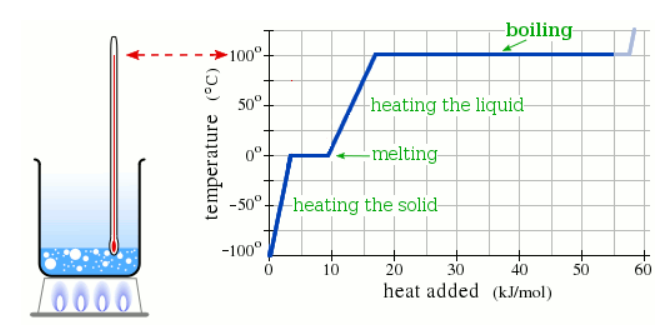
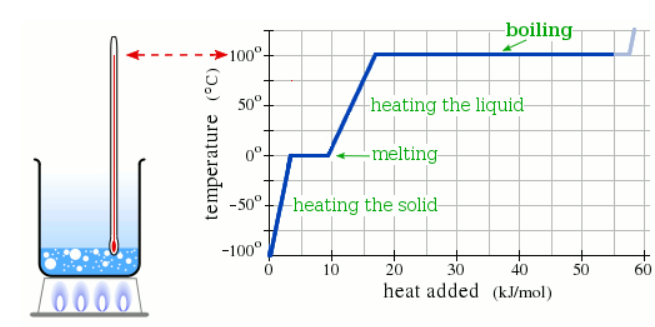
If a sample of pure X is a gas, in which region must the temperature and pressure be?
H
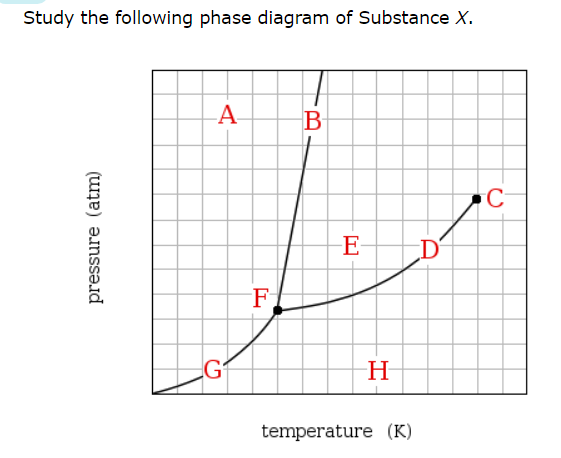
Which line must the temperature and pressure have crossed if a gaseous sample of X is observed to condense?
D
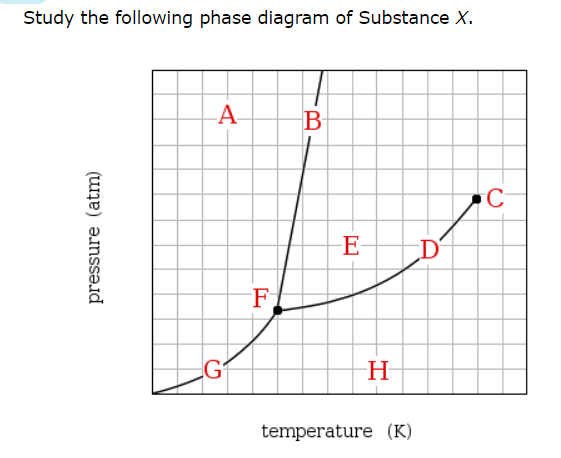
At which point would a sample of pure X be a mixture of solid, liquid and gas?
F
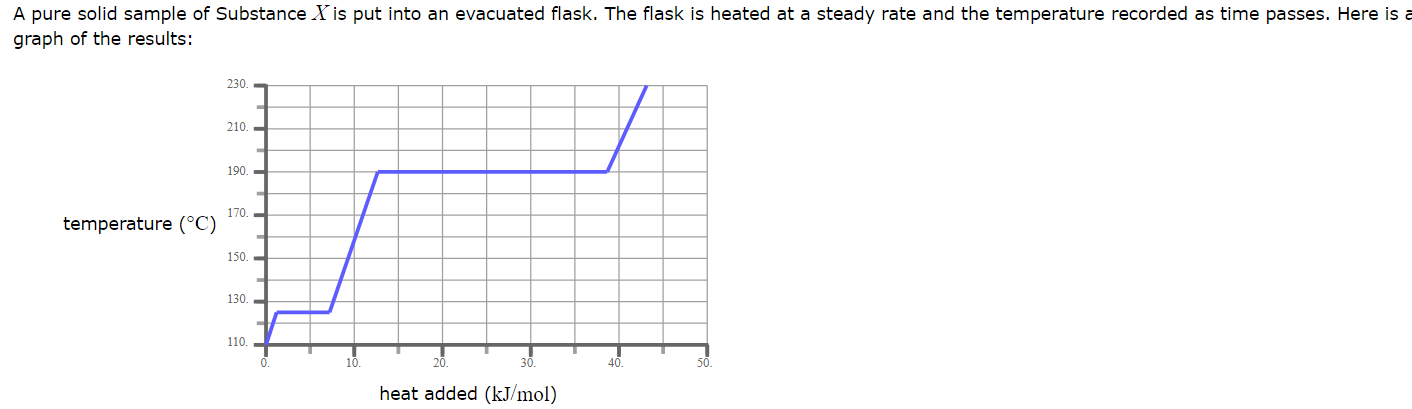
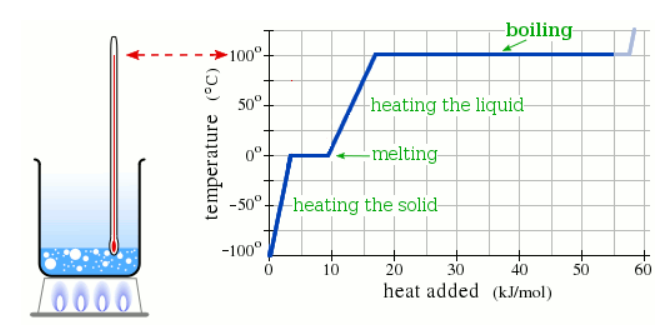
What is the boiling point of X ?
190.0 °C
What phase (physical state) of X would you expect to find in the flask after 10 kJ/mol of heat has been added?
Liquid
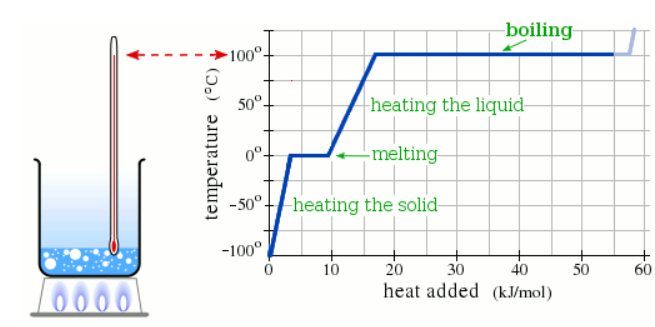
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results:
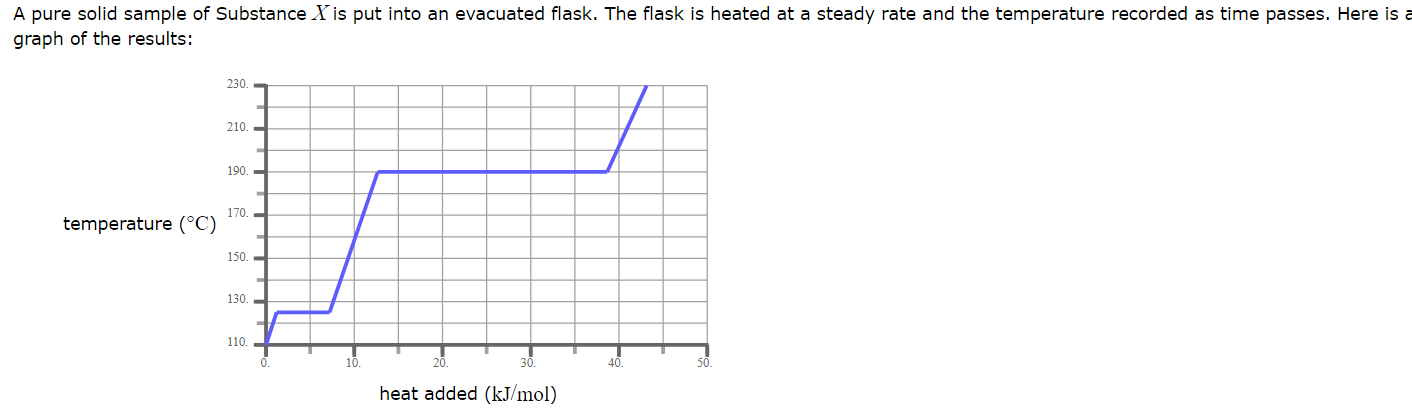
What is the melting point of X?
110.0 °C
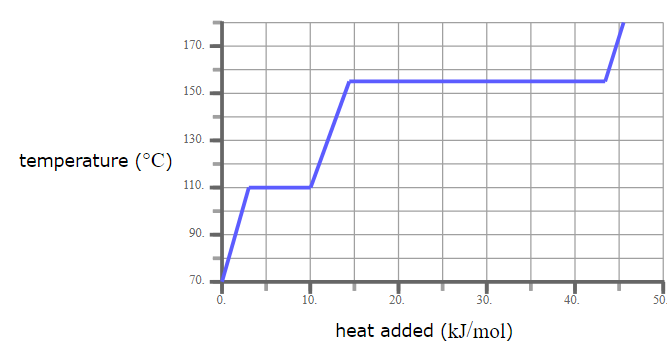
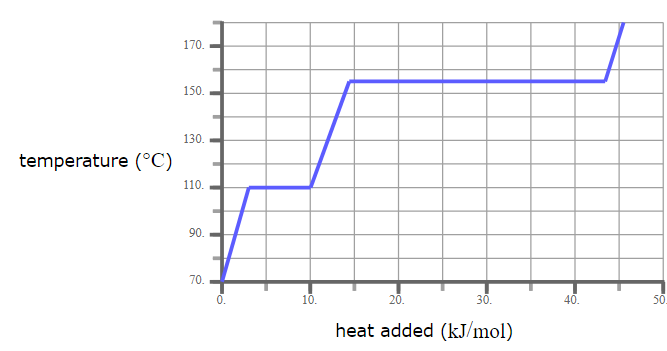
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results:
What phase (physical state) of X would you expect to find in the flask after 38 kJ/mol of heat has been added?
Liquid and Gas
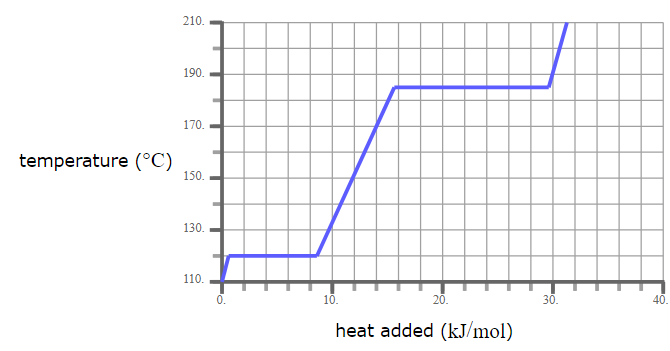
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results:
What is the melting point of X?
120.0 °C
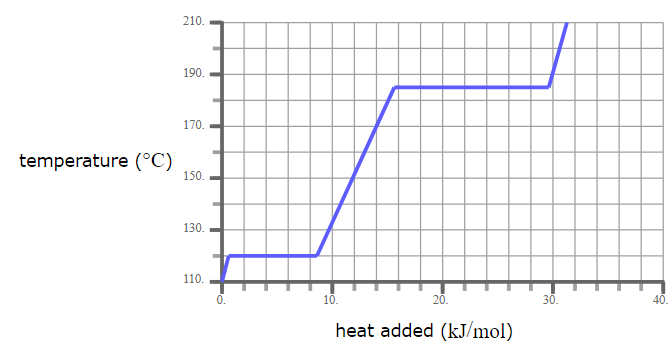
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results:
What phase (physical state) of X would you expect to find in the flask after 26 kJ/mol of heat has been added?
Liquid and Gas
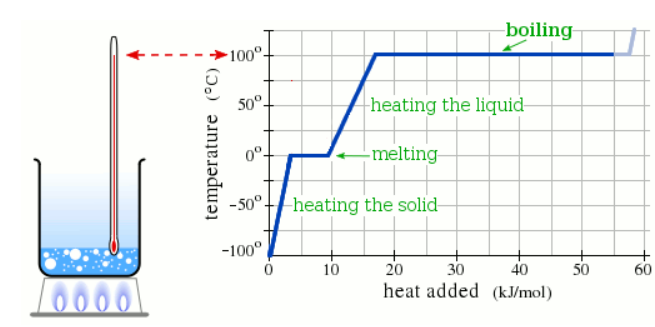
Observation
The enthalpy of vaporization of Substance E is bigger than that of Substance F.
Question
At any temperature where both substances are liquid, which has the higher vapor pressure?
Substance E
Substance F
Neither, E and F have the same vapor pressure.
It’s impossible to know without more information.
Substance F
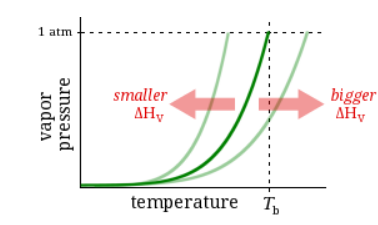
Observation
At 1atm pressure, Substance C boils at 88 °C and Substance D boils at 127 °C.
Question
Which has a higher enthalpy of vaporization?
Substance C
Substance D
Neither, C and D have the same enthalpy of vaporization.
It’s impossible to know without more information.
Substance D
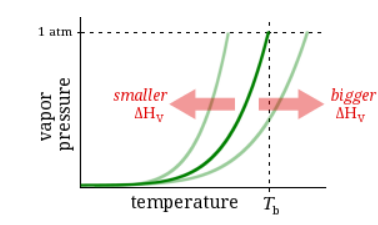
Observation:
At 64°C, Substance A has a vapor pressure of 132 torr and Substance B has a vapor pressure of 182 torr.
Question:
Which has a higher boiling point?
Substance A
Substance B
Neither, A and B have the same boiling point.
It’s impossible to know without more information.
Substance A
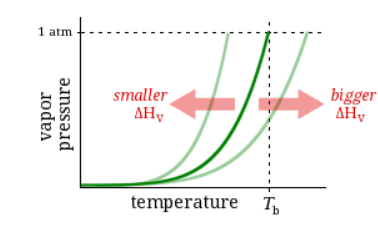
Observation:
At 1 atm pressure, Substance E boils at 78 °C and Substance F boils at 100 °C.
Question:
Which has a higher vapor pressure?
Substance E
Substance F
Neither, E and F have the same vapor pressure.
It’s impossible to know without more information.
Substance E
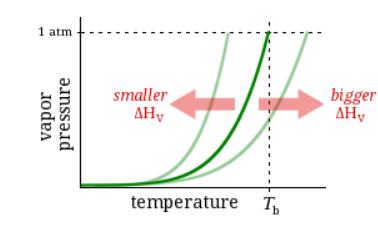
Observation:
At 44°C, Substance C has a vapor pressure of 115 torr and Substance D has a vapor pressure of 105 torr.
Question:
Which has a higher boiling point?
Substance C
Substance D
Neither, C and D have the same boiling point.
It’s impossible to know without more information.
Substance D
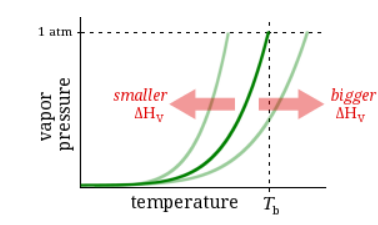
Observation
The enthalpy of vaporization of Substance A is smaller than that of Substance B.
Question
At any temperature where both substances are liquid, which has the higher vapor pressure?
Substance A
Substance B
Neither, A and B have the same vapor pressure.
It’s impossible to know without more information.
Substance A
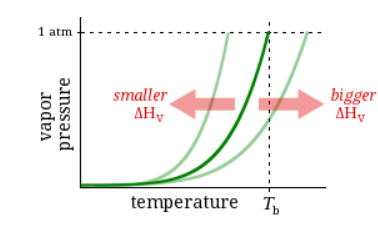
Observation
The enthalpy of vaporization of Substance C is bigger than that of Substance D.
Question
Which has the higher boiling point?
Substance C
Substance D
Neither, C and D have the same boiling point.
It’s impossible to know without more information.
Substance C
Higher enthalpy of vaporization → Higher boiling point
Observation
At 87°C, Substance E has a vapor pressure of 113 torr and Substance F has a vapor pressure of 123 torr.
Question
Which has a higher enthalpy of vaporization?
Substance E
Substance F
Neither, E and F have the same enthalpy of vaporization.
It’s impossible to know without more information.
Substance E
Higher enthalpy of vaporization → Higher boiling point → Lower vapor pressure → Higher enthalpy of vaporization
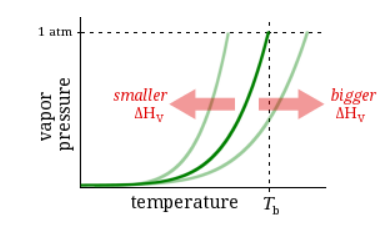
Observation:
At 1 atm pressure, Substance E boils at 78 °C and Substance F boils at 100 °C.
Question:
Which has a higher enthalpy of vaporization?
Substance E
Substance F
Neither, E and F have the same enthalpy of vaporization.
It’s impossible to know without more information.
Substance F
Observation
At 81°C, Substance C has a vapor pressure of 119 torr and Substance D has a vapor pressure of 79 torr.
Question
Which has a higher boiling point?
Substance C
Substance D
Neither, C and D have the same boiling point.
It’s impossible to know without more information.
Substance D
Observation
The enthalpy of vaporization of Substance C is smaller than that of Substance D.
Question
At any temperature where both substances are liquid, which has the higher vapor pressure?
Substance C
Substance D
Neither, C and D have the same vapor pressure.
It’s impossible to know without more information.
Substance C
Observation:
At 1 atm pressure, Substance A boils at 62 °C and Substance B boils at 35 °C.
Question:
Which has a higher enthalpy of vaporization?
Substance A
Substance B
Neither, A and B have the same enthalpy of vaporization.
It’s impossible to know without more information.
Substance A
Observation
At 60 °C, Substance E has a vapor pressure of 141 torr and Substance F has a vapor pressure of 131 torr.
Question
Which has a higher enthalpy of vaporization?
Substance E
Substance F
Neither, E and F have the same boiling point.
It’s impossible to know without more information.
Substance F
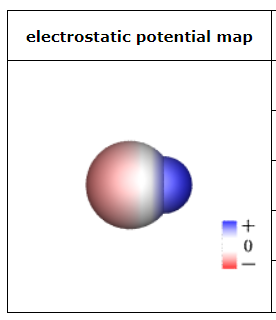
This sketch of a neutral molecule is shaded red or blue wherever the electrostatic potential at the molecule's surface isn't zero. What could the chemical formula of the molecule be?
Cl2
OCS
HBr
F2
ClF
HBr
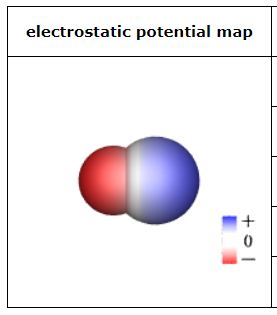
This sketch of a neutral molecule is shaded red or blue wherever the electrostatic potential at the molecule's surface isn't zero. What could the chemical formula of the molecule be?
HCl
H2CO
HCN
BrF
Cl2
BrF
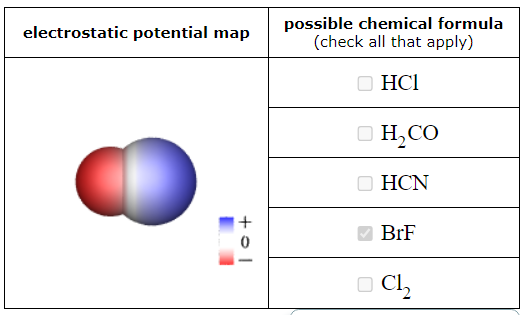
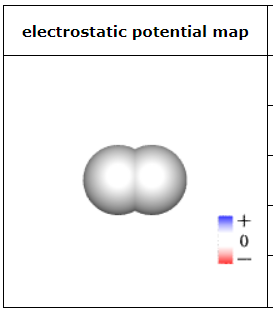
This sketch of a neutral molecule is shaded red or blue wherever the electrostatic potential at the molecule's surface isn't zero. What could the chemical formula of the molecule be?
SO2
HCl
ClF
F2
HI
F2
What are the intermolecular forces present in silicon tetrafluoride?
dispersion
dipole
hydrogen-bonding
dispersion
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, silicon tetrafluoride is a nonpolar molecule.
Hydrogen bonding: No, silicon tetrafluoride does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in dichloromethane (CH2Cl2)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, dichloromethane is a polar molecule due to the electronegativity difference between carbon, hydrogen, and chlorine atoms.
Hydrogen bonding: No, dichloromethane does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine, which is a requirement for hydrogen bonding.
What are the intermolecular forces present in hypobromous acid (HBrO)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, hypobromous acid is a polar molecule due to the electronegativity difference between hydrogen, bromine, and oxygen atoms.
Hydrogen bonding: Yes, hypobromous acid has a hydrogen atom bonded to oxygen, which can form hydrogen bonds.
What are the intermolecular forces present in carbonyl sulfide (COS)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
Since the dispersion force acts between any two molecules, you should check the "dispersion" box for all substances.
Since carbonyl sulfide (COS) is made of polar molecules, you should check the "dipole" box.
What are the intermolecular forces present in hydrogen bromide?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Since the dispersion force acts between any two molecules, you should check the "dispersion" box for all four substances.
Since hydrogen bromide (HBr) is made of polar molecules, you should check the "dipole" box.
What are the intermolecular forces present in ammonia?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Since the dispersion force acts between any two molecules, you should check the "dispersion" box for all substances.
Since ammonia (NH3) is made of polar molecules, you should check the "dipole" box.
Since ammonia (NH3) is made from molecules that can form a hydrogen-bonding arrangement with each other, you should check the "hydrogen-bonding" box.

What are the intermolecular forces present in carbon disulfide?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Since the dispersion force acts between any two molecules, you should check the "dispersion" box for all substances.
What are the intermolecular forces present in nitrogen tribromide?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, nitrogen tribromide (NBr3) is a polar molecule due to the electronegativity difference between nitrogen and bromine atoms.
Hydrogen bonding: No, nitrogen tribromide does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in water?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, water is a polar molecule due to the electronegativity difference between hydrogen and oxygen atoms.
Hydrogen bonding: Yes, water has a hydrogen atom bonded to oxygen, which can form hydrogen bonds.
What are the intermolecular forces present in fluoromethane (CH3F)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, fluoromethane is a polar molecule due to the electronegativity difference between carbon, hydrogen, and fluorine atoms.
Hydrogen bonding: No, fluoromethane does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine, which is a requirement for hydrogen bonding.
What are the intermolecular forces present in difluoromethane (CH2F2)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, difluoromethane is a polar molecule due to the electronegativity difference between carbon, hydrogen, and fluorine atoms.
Hydrogen bonding: No, difluoromethane does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in nitrogen (N2)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, nitrogen is a nonpolar molecule.
Hydrogen bonding: No, nitrogen does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in hypochlorous acid (HClO)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, hypochlorous acid is a polar molecule.
Hydrogen bonding: Yes, hypochlorous acid has a hydrogen atom bonded to oxygen, which can form hydrogen bonds.
What are the intermolecular forces present in hydrogen chloride?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, hydrogen chloride (HCl) is a polar molecule due to the electronegativity difference between hydrogen and chlorine atoms.
Hydrogen bonding: No, hydrogen chloride does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine, which is a requirement for hydrogen bonding.
What are the intermolecular forces present in hydrogen fluoride?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, hydrogen fluoride (HF) is a polar molecule due to the electronegativity difference between hydrogen and fluorine atoms.
Hydrogen bonding: Yes, hydrogen fluoride has a hydrogen atom bonded to fluorine, which can form hydrogen bonds.
What are the intermolecular forces present in chloromethane (CH3Cl)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, chloromethane is a polar molecule due to the electronegativity difference between carbon, hydrogen, and chlorine atoms.
Hydrogen bonding: No, chloromethane does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in bromine (Br2)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, bromine is a nonpolar molecule.
Hydrogen bonding: No, bromine does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in chlorine (Cl2)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, chlorine is a nonpolar molecule.
Hydrogen bonding: No, chlorine does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in hydrogen (H2)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, hydrogen is a nonpolar molecule.
Hydrogen bonding: No, hydrogen does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in formaldehyde (CH2O)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, formaldehyde is a polar molecule due to the electronegativity difference between carbon, hydrogen, and oxygen atoms.
Hydrogen bonding:
Yes, formaldehyde has a hydrogen atom bonded to oxygen, which can form hydrogen bonds. NO
What are the intermolecular forces present in carbon monoxide?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, carbon monoxide (CO) is a polar molecule due to the electronegativity difference between carbon and oxygen atoms.
Hydrogen bonding: No, carbon monoxide does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in methane (CH4)?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, methane is a nonpolar molecule.
Hydrogen bonding: No, methane does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What are the intermolecular forces present in dichlorine monoxide?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: Yes, dichlorine monoxide (Cl2O) is a polar molecule due to the electronegativity difference between chlorine and oxygen atoms.
Hydrogen bonding: No, dichlorine monoxide does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine, which is a requirement for hydrogen bonding.
What are the intermolecular forces present in carbon tetrachloride?
dispersion
dipole
hydrogen-bonding
dispersion
dipole
hydrogen-bonding
Dispersion forces: Yes, these are present in all molecules.
Dipole-dipole interactions: No, carbon tetrachloride is a nonpolar molecule.
Hydrogen bonding: No, carbon tetrachloride does not have a hydrogen atom bonded to nitrogen, oxygen, or fluorine.
What intermolecular force is felt by all molecules?
Dispersion

What intermolecular force is felt by only polar molecules?
Dipole

What intermolecular force is felt by molecules that can form the special three-atom hydrogen bonding interaction?
Hydrogen-bonding

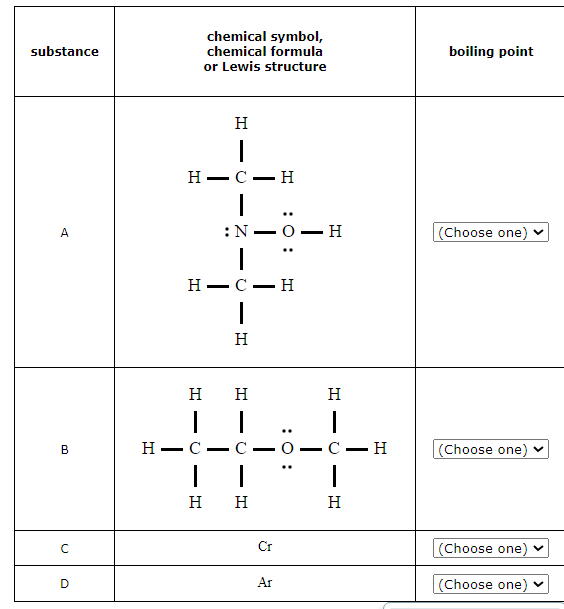
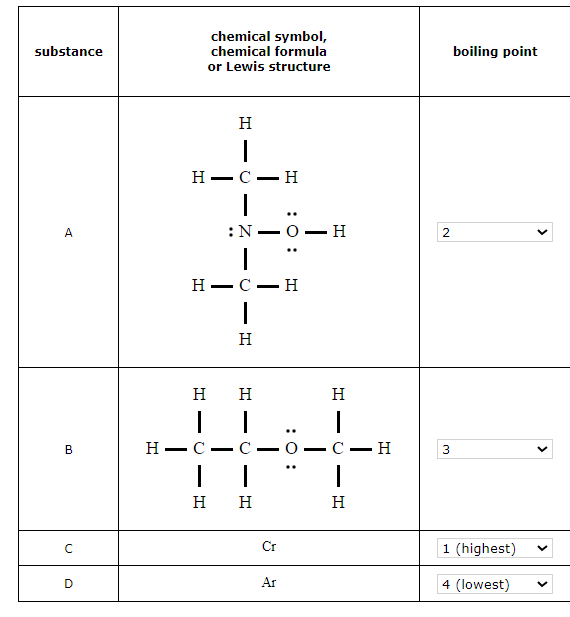
Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on.
1. Identify any substances with strong bonding forces in the liquid state.
The most important are metal elements and binary ionic compounds. The very strong bonding forces holding these substances together in the liquid state gives them very high boiling points.
In this case, the element chromium Cr is a metal. The very strong metal bonding forces between Cr atoms in the liquid state will give this substance the highest boiling point.
2. Compare molar masses.
When the molar masses of nonmetal elements or molecular compounds are not close, the substance with the highest molecular mass will usually have the highest boiling point. That's because the atoms or molecules in it will exert the strongest dispersion force on each other.
In this case, the molar masses of CH32NOH (61. g/mol) and CH3CH2OCH3 (60. g/mol) are significantly higher than the molar mass of Ar (40.g/mol), so we can expect CH32NOH and CH3CH2OCH3 to have higher boiling points than Ar.
Note: when there is a significant difference in molar mass, the difference in the strength of the dispersion force is usually more important than whether the molecules experience any special intermolecular forces, such as the dipole or hydrogen-bonding force.
3. For molecular compounds with similar molar mass, identify any special intermolecular forces.
Compounds made of molecules that exert the dipole or hydrogen-bonding force on each other will boil at higher temperatures than compounds made of similar-sized molecules that only exert the dispersion force.
In this case, the molar masses of CH32NOH (61.g/mol) and CH3CH2OCH3 (60.g/mol) are similar, but CH32NOH molecules can form hydrogen bonds with each other while CH3CH2OCH3 molecules can't. The extra hydrogen-bonding force the CH32NOH molecules exert on each other will make the boiling point of CH32NOH higher.
For compounds made of molecules that are very similar—comparable molar mass, and the same special intermolecular forces—you may need to look at finer details, such as the shape of each molecule, how polar each molecule may be, or the number of hydrogen bonds each molecule might form. In this problem, a closer look isn't necessary.
You can now order the compounds according to their boiling points.