5) Ionic radii and ionic substitution
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
what is the relationship between atomic radius and the periodic table
decreases from left to right
increases from top to bottom
what properties are influenced by ionic radii
substitution of elements in solids
solubility
diffusion rates
what are factors affecting ionic radii
type of ion
magnitude of charge
coordination number
how does type of ion affect ionic radius
cations are smaller because they have lost an electron
anions are larger because they have gained an electron
how does magnitude of charge affect ionic radius
as charge increases in cations (+1 to +2), they become smaller (losing more electrons)
as charge increases in anions (-1 to -2), they become larger (gaining more electrons)
what is coordination number? what is it determined by?
number of oppositely charged ions an ion is bonded to in a compound
if the cation is very small, the number of anions you can pack around it without overlapping. If the cation is larger, you can fit more anions around
determined by the relative radius of the cation an anion: radius of cation / radius of anion = radius ratio (higher ratio = higher coord number)
oxygen is used as the anion, most common anion in silicates
explain radius ratio categories
as cation radius increases, radius ratio increases
CN [3] = triangular, [4] = tetrahedral coord, [6] = octahedral (8faces), [8] = cubic (corners of cube), [12] = cubooctahedron (close packing)
all silicates have silicon in tetrahedral coord, but there are also other elements in others coords and other structural sites
what is the effect of coord number on ionic radii
if magnesium needs to go into a tetrahedral site, which it doesn’t usually, it has to shrink, and if it goes into octahedral site, it will expand
ionic radii increases with coord number
explain relationship between coord number with oxygen in silicates and the particular ion
+1 have higher coord numbers (they are bigger, can accommodate more elecs), higher charge numbers are are [4] (they are smaller, can’t accommodate as many elecs)
explain bond length
distance between nucleii
when 2 atoms are far apart, there is no energetic attraction between them, when they get closer, negative energy facilitates bond formation
when they are too close, nucleii start repulsing each other
maximum negative attractive energy will be where a bond will happen (that’s the bond length), closest they can be without the repulsive force taking over
bond length is radius of one atom to another
explain compositional variation in mineral with example
minor and trace elements substitute for major elements in crystal structure
minerals are solid solution
ex: MgSiO3 (Opx),
bottom left side is recalculated atomic proportions, calculate cations base on a fixed number of oxygens (olivine its 4, feldspar its 8)
olivine either has lots of Mg or lots of Fe, plus Silicon, so total cations should add to 3 (2 of either Mg or Fe, and silicon)
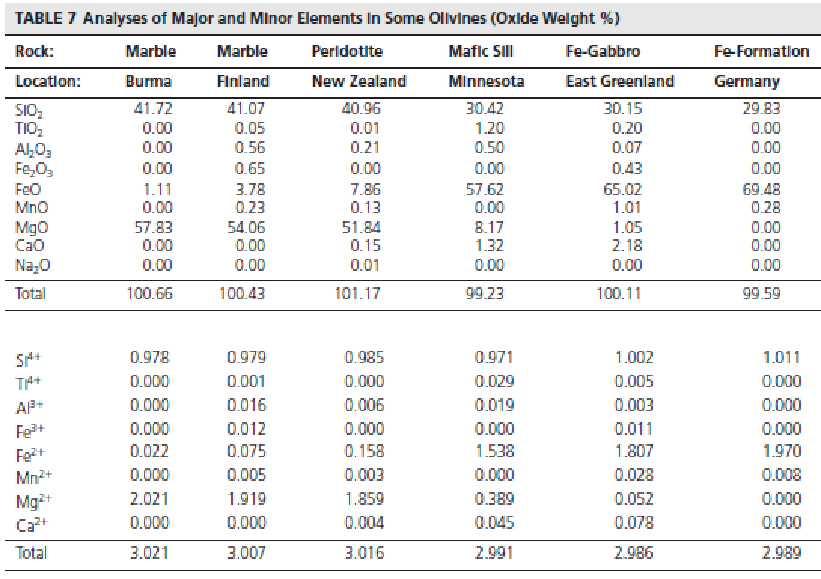
what are the coord numbers for the elements in olivine
silicon: tetrahedral
Fe and Mg: octahedral, M1 and M2 (M2 is bigger)
what goldschmidts rule (words)
same size (or close): has to occupy same physical space
similar charges: minerals as a whole are neutral
higher ionic potential: charge/radius of the cations forms a stronger bond with anions surrounding the site (higher the charge, more charge density, will make a stronger bond than an atom with lower charge)
electroneg of ions need to be similar
similar charge and size, smaller ion will be in solid
similar size, diff charge, higher charge will be in solid
how to calculate relative size difference for goldschmidt’s rule
relative size difference = difference in ionic radii / radii of original ion in site *100
<15%: complete substitution, easy
15-30%: limited substitution, some
>30%: no substitution possible
how does temperature affect ionic substitution
ions of dissimilar sizes can substitute easier at high temps, higher temp causes more flexibility in the site to incorporate a slightly different size
how does pressure affect ionic substitution
higher pressures cause more rigid structures, smaller cation can be preferentially accommodated in a spot with less space
not as significant as temp
what are the types of ionic substitutions
simple substitution
coupled substitution
interstitial solid solution
omission solid solution
explain simple substitution, example
ionic substitution between ions of similar size and same charge
Mg, Fe, Mn substitution (Mg smallest)
explain coupled substitution, example
ions are similar size but different charge (one off), need another substitution along with original to maintain charge balance
Na and Ca, Al and Si, Na(albite) and Ca (anorthite)
Si (+4) and Na (+) => Ca (2+) and Al (3+)
Albite: NaAlSi3O8, Anorthite: CaAl2Si2O8
explain interstitial solid solution, example, what keeps the atom there?
ion occupies the interstitial site instead of a regular crystallographic site
carbon steel (interstitial): carbon sits between the iron atoms
brass (not interstitial)
Beryl: Na sits inside the beryl ring, not the regular cryst sites
what keeps atom there: weak bonding, residual charges in a certain area of the molecule that the cation can weakly bond to, would be easily dislodged if you were to heat it up
explain omission solid solution, example?
substitution of highly charged ions resulting in leaving empty cryst sites to maintain charge balance
K-feldspar: potassium replaced with lead (K+ + K+ → Pb2+ + vacancy
this is considered a defect, can’t have too many of these or the mineral structure becomes unstable (can’t have a lead endmember of feldspar)
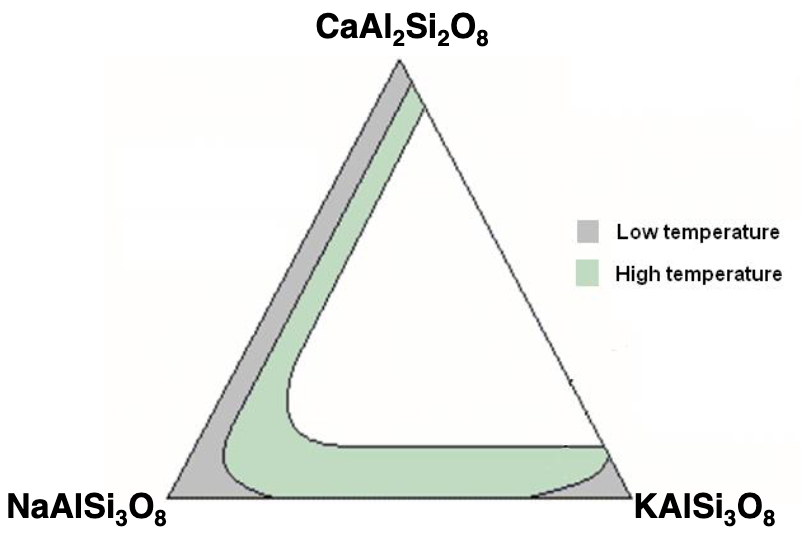
explain this graph Ca = An, Na = Ab, K = Orth
shows the possible range of feldspar substitution (grey = low temp, green = high temp)
grey: Albite - anorthite: complete solid solution, coupled substitution, 100Ca, 100Na, and all in between
grey: albite - orthoclase: amount of Na you can put in orthoclase is limited because the size is much different, same with putting K in albite, only partial/limited substitution, not complete solid solution
grey: anorthitite - orthoclase: different size and different charges, can hardly do any substitution
green: albite-orthoclase: higher temp allows Na and K to more easily substitute, creating solid solution
green: albite-anorthite: plagioclase has solid solution at any temp, but high temp can have a bit more potassium, as it cools that forms exsolution
green: anorthite-orthoclase: size and charge difference still prevents complete solid solution