Clinical biomedicine
1/307
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
308 Terms
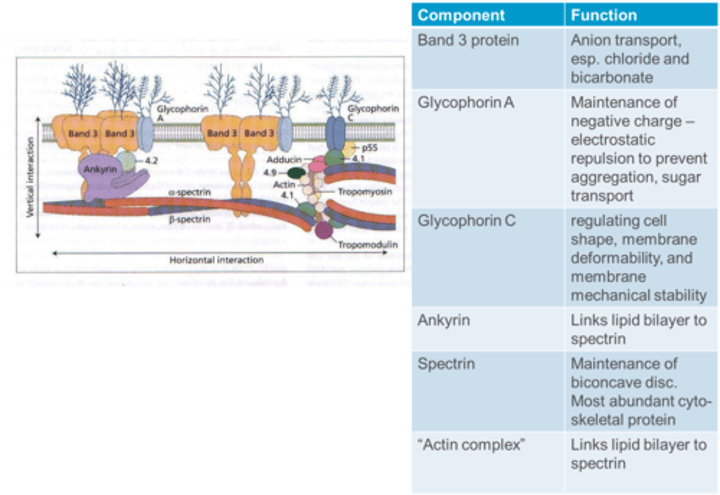
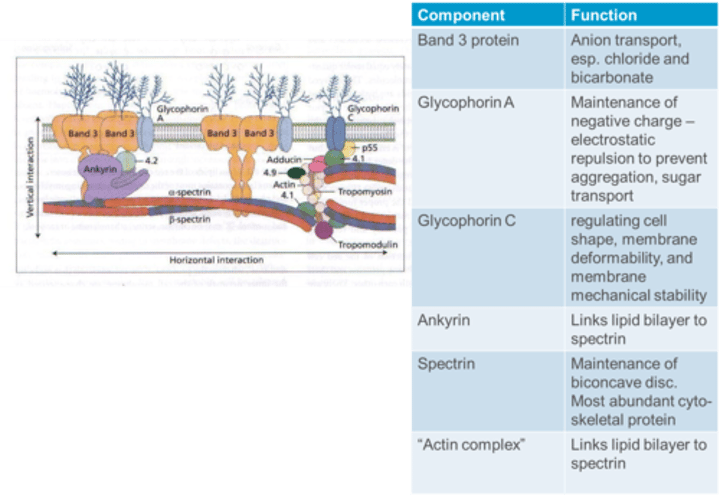
What is the function of Glycophorin C in the RBC membrane?
Glycophorin C is essential for regulating cell shape, membrane deformability, and membrane mechanical stability.
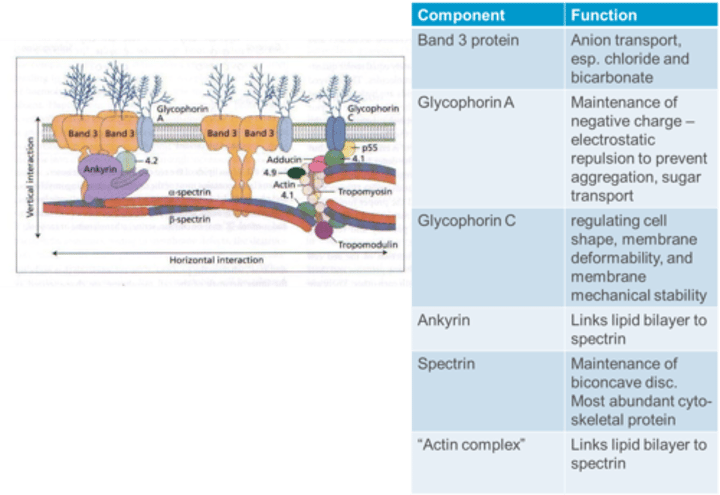
What is the function of Ankyrin in the RBC membrane?
Ankyrin links the lipid bilayer to spectrin in the RBC membrane.
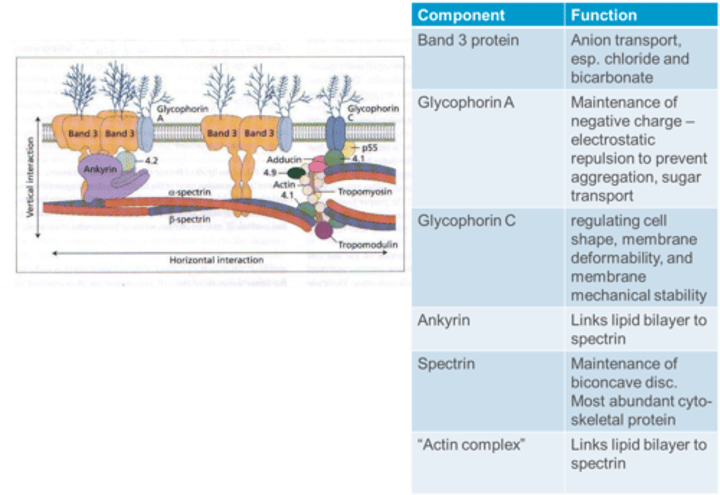
What is the function of Spectrin in the RBC membrane?
Spectrin is crucial for the maintenance of the biconcave disc shape and is the most abundant cytoskeletal protein in the RBC membrane.
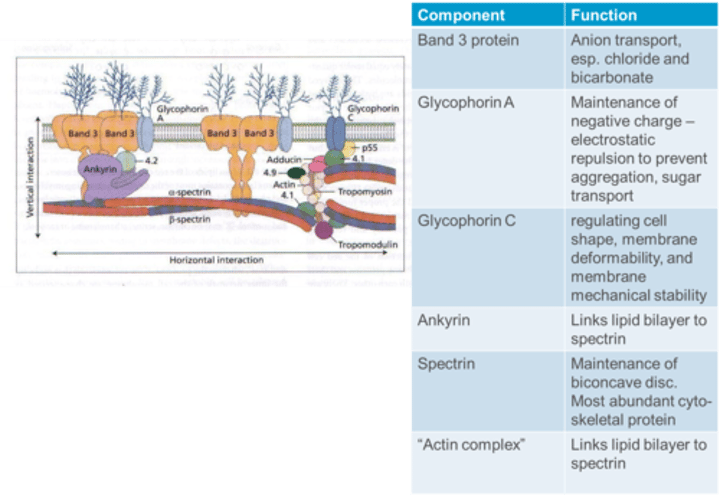
What is the function of the "Actin complex" in the RBC membrane?
The "Actin complex" links the lipid bilayer to spectrin, contributing to membrane stability in the RBC.
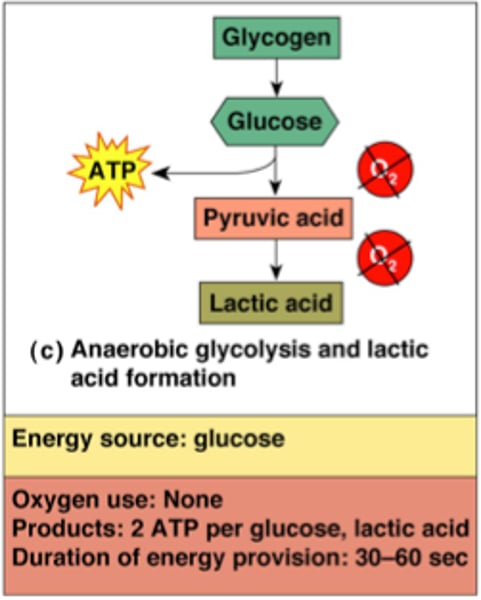
What is the primary metabolic activity in RBCs?
RBCs primarily engage in anaerobic glycolysis to maintain Haem Fe in the reduced state (Fe2+) using NADPH from the pentose phosphate pathway.
Oxidation to Fe3+ causes Hemoglobin (Hb) to become MetHb, rendering it inoperative as an oxygen carrier.
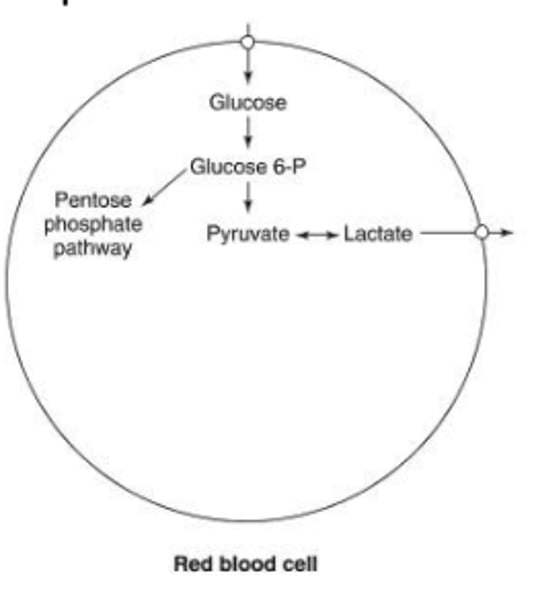
Why is the production of 2,3 DPG (2,3 BPG) important in RBC metabolism?
The production of 2,3 DPG (2,3 BPG) is crucial to regulate oxygen affinity in RBCs.
How does anaerobic glycolysis contribute to RBC metabolism?
Anaerobic glycolysis is essential for producing ATP, serving as a source of energy to maintain cell membrane deformability and regulate ion and water exchange in RBCs.
How many red blood cells are destroyed and replaced per day?
Approximately 10^11 RBCs (nearly 1 million every second)
By how much can red blood cell production be increased?
RBC production can be upregulated by 10 times
What is the typical range of red blood cells in 1mm3 of blood?
3.5 - 5.5 x 10^6 RBCs
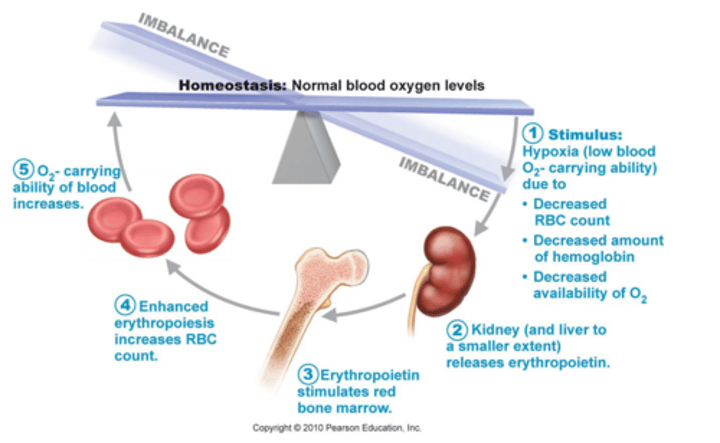
What is the role of Erythropoietin (Epo) in red cell production?
Erythropoietin (Epo) acts on committed erythroid precursors to increase cell division, consequently increasing the number of red blood cells (RBCs).
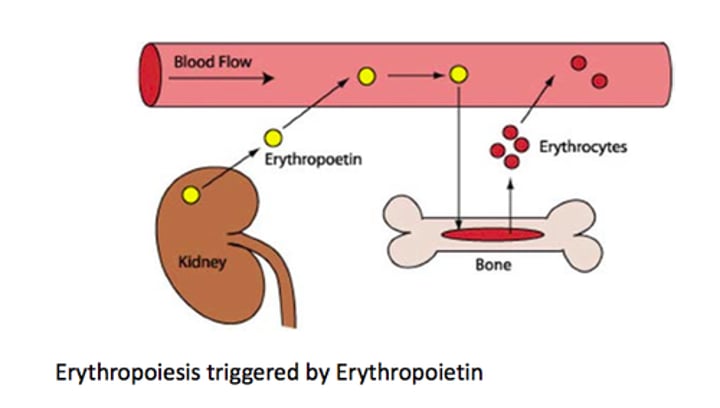
What stimulates the production of Erythropoietin (Epo)?
Renal hypoxia (low oxygen levels) is a stimulus that increases Erythropoietin (Epo) production.
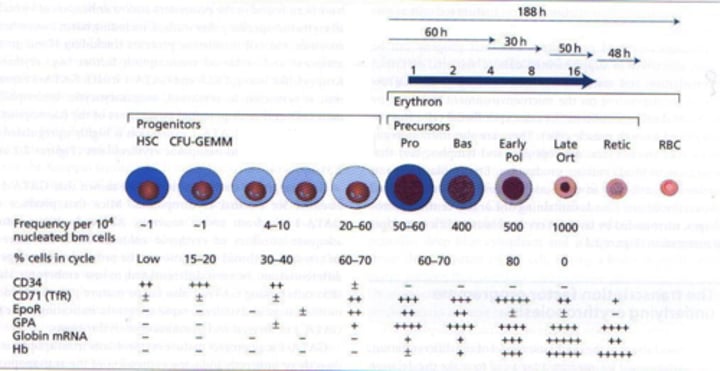
What happens to RBC nuclei during their production?
RBC nuclei are extruded and phagocytosed before the release of mature RBCs from the bone marrow.

What occurs in the cytoplasmic organelles of reticulocytes (immature RBCs) after release into circulation?
The cytoplasmic organelles, including mitochondria and ribosomes, of reticulocytes continue to synthesize hemoglobin for 1-2 days after release into circulation.
Red cell production is regulated by
Erythropoetin (Epo), produced in the kidneys
What is an immature nucleated RBC called?
a normoblast
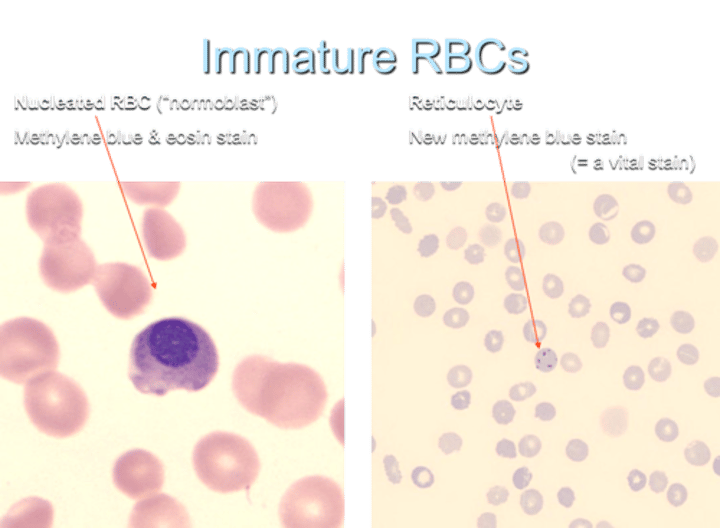
What is the term for an immature non-nucleated RBC?
a reticulocyte
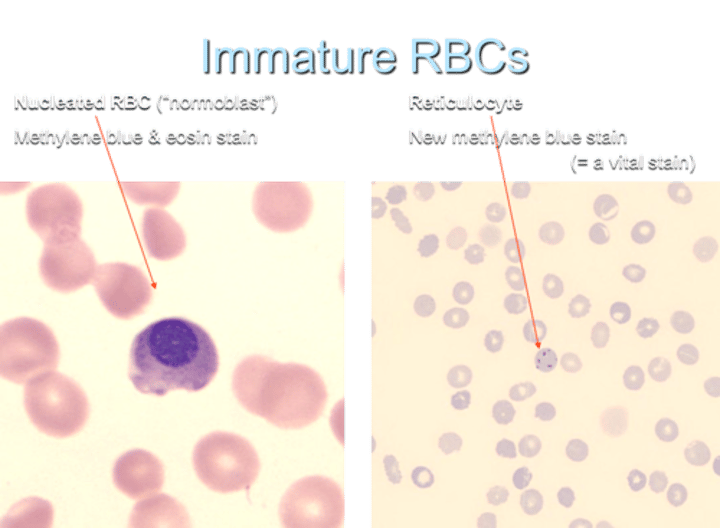
RBC lifespan?
What is a shortening of the lifespan called
Usually approx 120 days
A shortening of the RBC lifespan = haemolysis. (May be compensated by increased production, so not necessarily anaemic - "compensated haemolysis")
Increased reticulocyte count =
increased rate of RBC production
(adult normal range 0.5 - 1.5%)
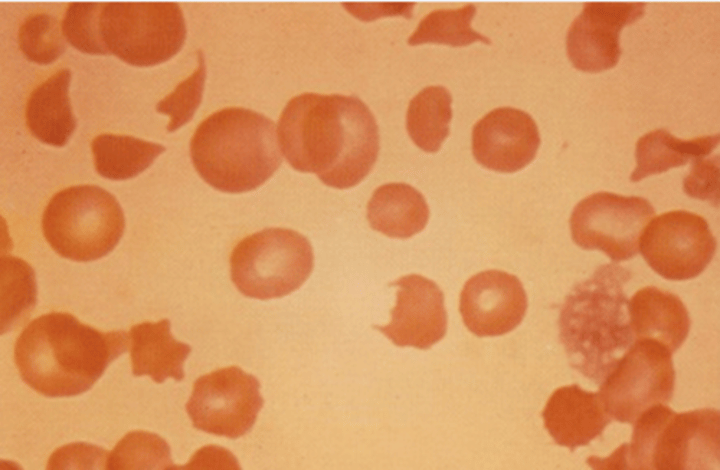
How are senescent or defective RBCs destroyed in the body?
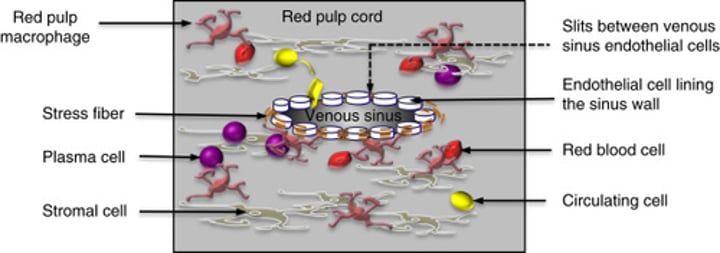
Macrophages in the reticuloendothelial (RE) system, especially in the spleen, are responsible for the destruction of senescent or defective RBCs.
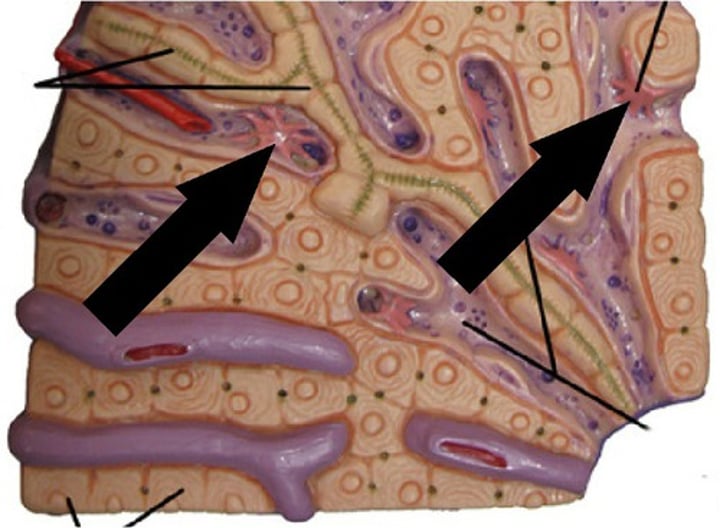
What are splenic cords?
found in the red pulp of the spleen between the sinusoids, consisting of fibrils and connective tissue cells with a large population of monocytes and macrophages.
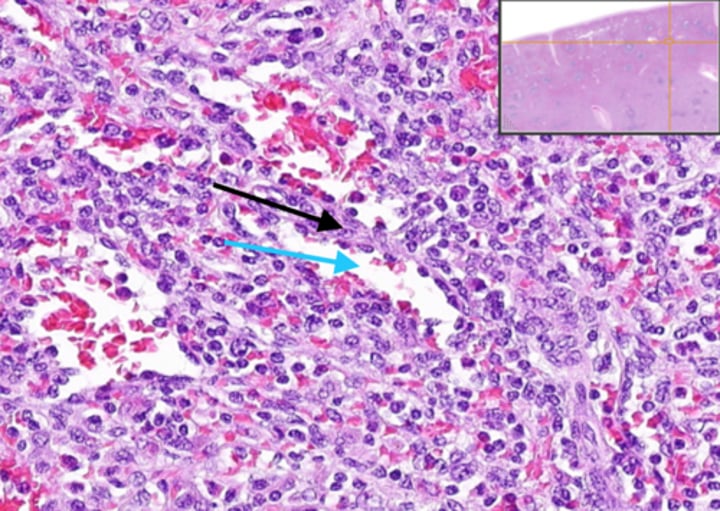
What distinguishes the spleen as a filtering organ for RBCs?
The spleen acts as a very discriminating filter with 3μ windows separating splenic cords from venous sinuses.
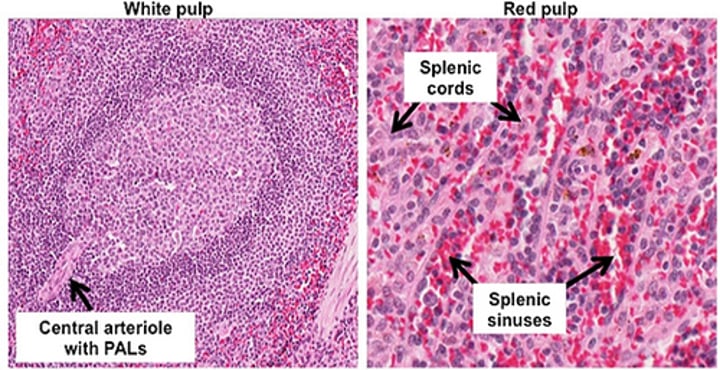
Describe the structure of splenic cords and venous sinuses.
Splenic cords are blind sacs between sinuses with no opening orifices into sinuses. Sinuses are blood spaces surrounded by fibrous "filter" structures.
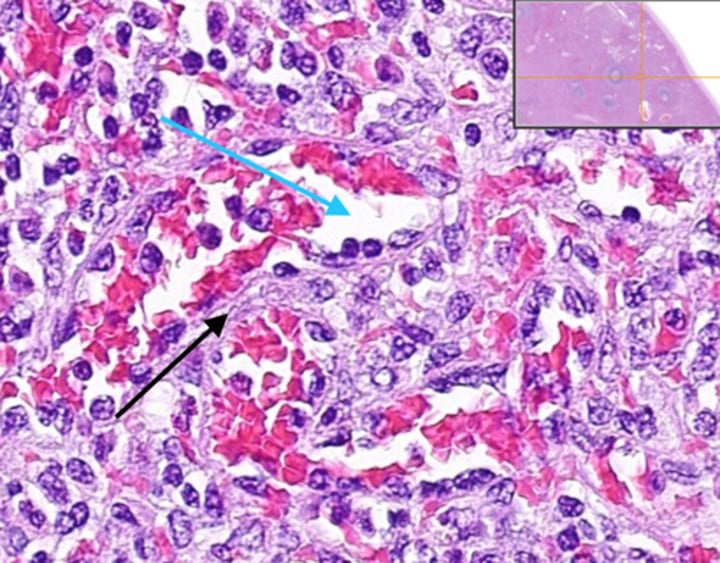
What cells line the splenic cords?
Macrophages line the splenic cords, participating in the filtering and removal of senescent or defective RBCs.
What factors hasten the destruction of RBCs by splenic macrophages in the red pulp?
Loss of membrane pliability or antibody coating can accelerate the destruction of RBCs by splenic macrophages in the red pulp.
What happens to iron during the destruction of RBCs in the spleen?
Iron is recycled by splenic macrophages during the destruction of RBCs.
What types of particles are removed by splenic macrophages during RBC destruction?
nuclear material (aka Howell-Jolly bodies)
cytoplasmic organelles,
Siderotic (= iron containing) granules,
oxidised Hb (methaemoglobin)
Why might reticulocytes experience slower passage through the spleen?
Reticulocytes may be "sticky," slowing their passage through the spleen.
What is the primary function of Hemoglobin (Hb)?
Haemoglobin is responsible for the transportation of oxygen from the lungs to respiring tissues.
Describe the appearance of Oxyhemoglobin.
Oxyhemoglobin, in its oxygenated state, exhibits a bright red color.
Describe the appearance of Deoxyhemoglobin.
Deoxyhemoglobin, in its deoxygenated state, appears dark red.
How is oxygen bound to hemoglobin?
Oxygen is reversibly bound to a heme group, which includes an Fe (iron) atom in a porphyrin ring. This interaction is physical, not chemical.
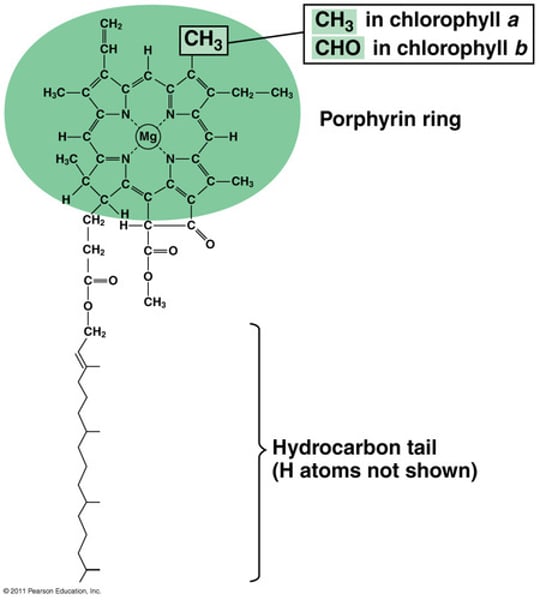
What happens to the Fe atom during oxygen binding?
The Fe atom remains in a reduced form during oxygen binding, preventing the formation of non-functional methemoglobin (MetHb) in its Fe3+ state.
What percentage of total CO2 transport from respiring tissue to the lungs is associated with RBCs?
RBCs are associated with about 95% of total CO2 transport from respiring tissue to the lungs.
How much of the transported CO2 is directly bound to Hemoglobin?
About 25% of the transported CO2 is directly bound to Hemoglobin, specifically to the N-terminal valine of α-chains.
What is the role of membrane-associated carbonic anhydrase in RBCs?
Membrane-associated carbonic anhydrase catalyzes the reaction:
CO2 + H2O → H2CO3- (carbonic acid),
which rapidly dissociates to H+ and HCO3-.
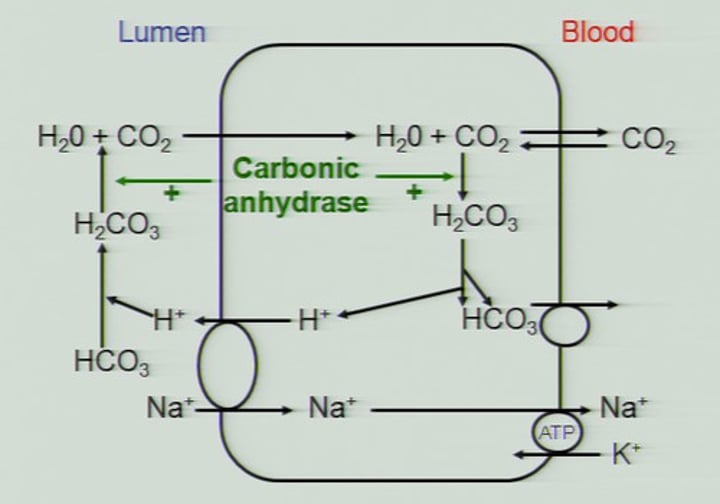
What happens to HCO3- after its formation in RBCs?
HCO3- diffuses back into the plasma, constituting about 70% of the transported CO2. air.
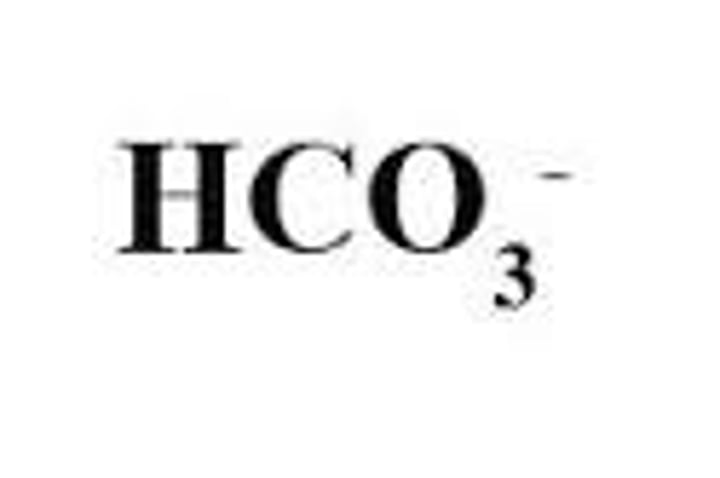
How is CO2 released in the alveoli during the process of CO2 transport?
HCO3- dissociates back into CO2 + H2O in the alveoli, and CO2 is released to the
What is another function of Hemoglobin (Hb) related to pH regulation?
Hemoglobin acts as a buffer. H+ ions, left from the dissociation of carbonic acid to bicarbonate, bind to globin chains of Hb.
How does Hb function as a buffer in the blood?
Hb acts as a buffer, helping maintain stable plasma pH. This action protects against respiratory acidosis, considering the physiological blood pH range is between 7.35 and 7.45.
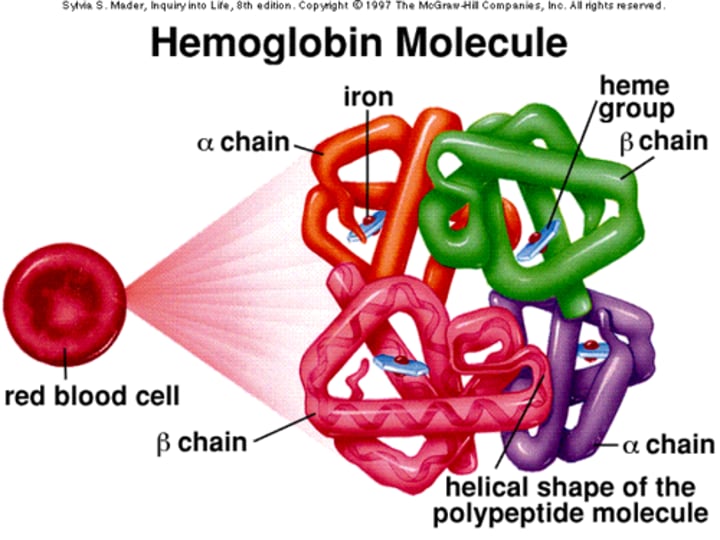
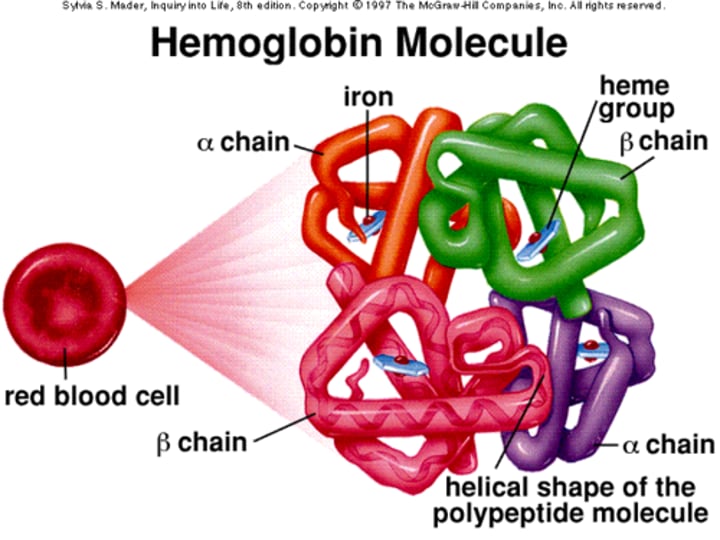
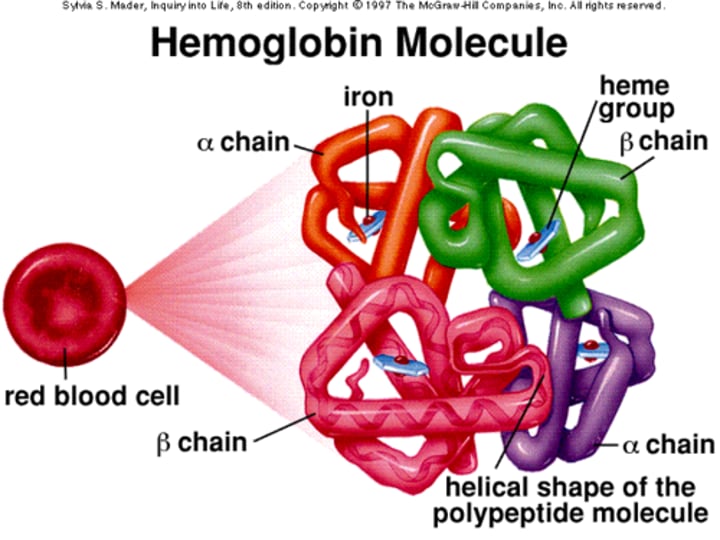
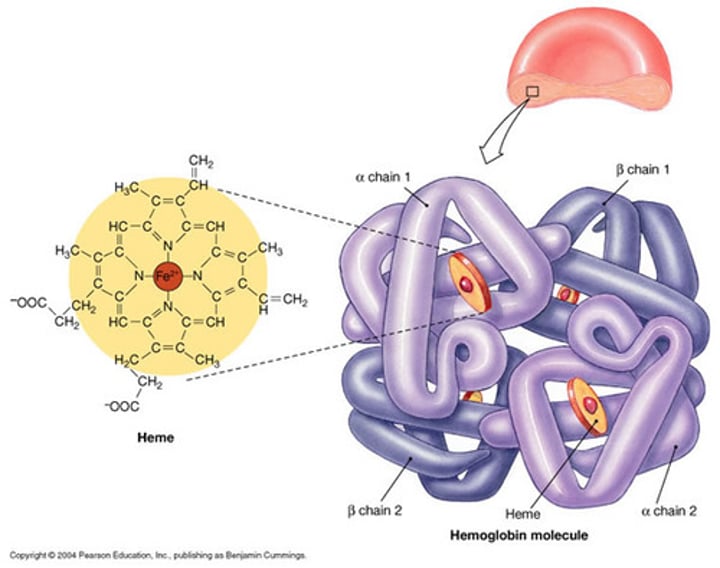
What is the structure of Hemoglobin (Hb) in terms of its molecular weight?
Hemoglobin is a tetrameric molecule with a molecular weight of about 68 kDa.
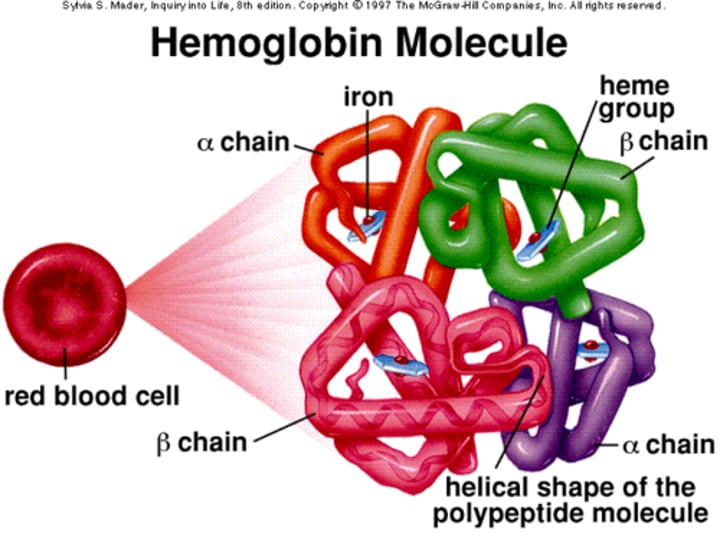
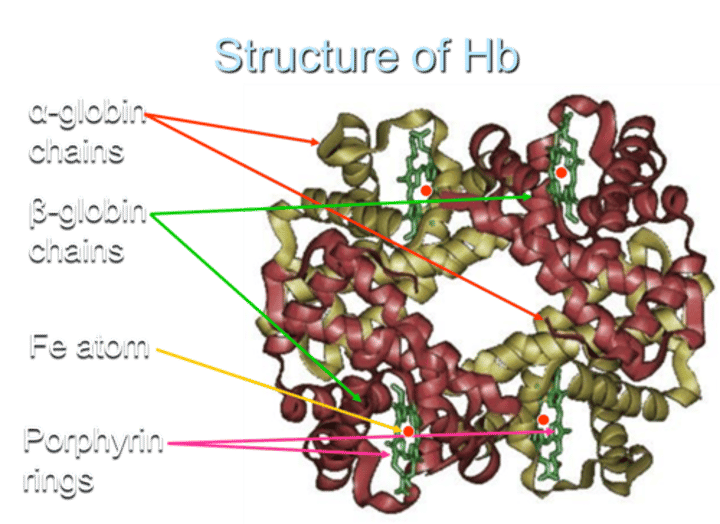
How many globin chains are present in each Hb molecule?
Each Hemoglobin (Hb) molecule consists of 4 globin chains of two types.
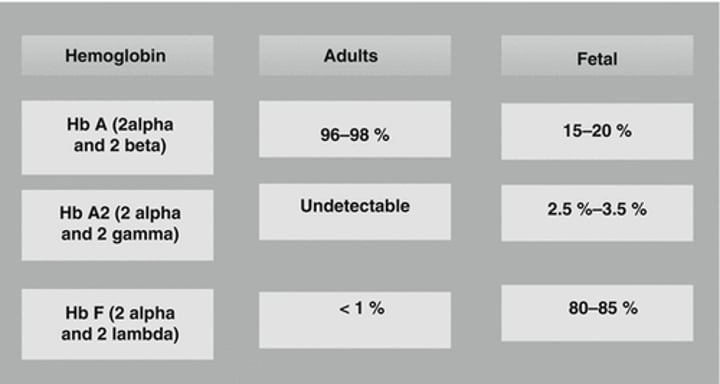
What are the types of globin chains in Adult Hemoglobin (HbA)?
Adult Hemoglobin (HbA) consists of two types of globin chains: α2ß2 and Hb A2 (α2δ2).
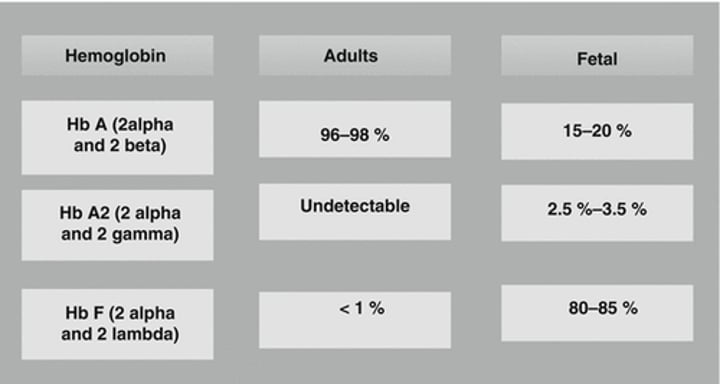
What is the type of globin chains in Fetal Hemoglobin (HbF)?
Fetal Hemoglobin (HbF) consists of α2γ2 globin chains.
How many Haem groups are present in each Hemoglobin (Hb) molecule?
Each globin chain surrounds a Haem ("prosthetic") group, and there are 4 haem groups in each Hemoglobin (Hb) molecule.
Structure of Hb
What is the relationship between Hemoglobin (Hb) structure and its function concerning O2 affinity?
The objective is to have a high O2 affinity where the partial pressure of oxygen (pO2) is high and low affinity where pO2 is low.
RBCs (Erythrocytes) basic structure
Biconcave disc
-Mean diameter 7μ,
-mean volume 78 - 101fl
No nucleus or cytoplasmic organelles in mature RBC
Contain Hb as major component (25% by volume and 33% by weight)
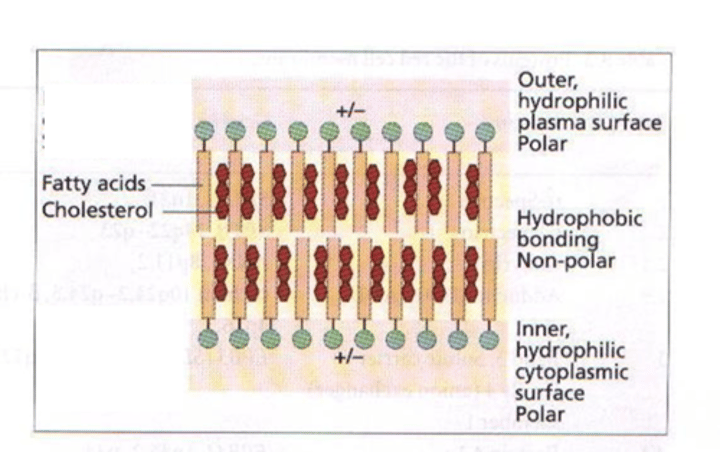
What is the red blood cell membrane made up of?
Membrane is a lipid bilayer, made up of 40% lipids, 52% protein, 8% carbohydrate.
Outer surface is negatively charged to stop RBCs aggregating
Red Cell cytoskeleton
Arrangement of some membrane proteins (“cytoskeleton”)
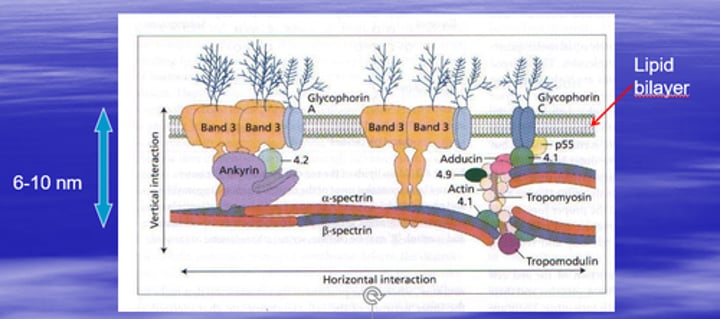
What is the function of Band 3 protein in the RBC membrane?
Band 3 protein is involved in anion transport, especially chloride and bicarbonate.
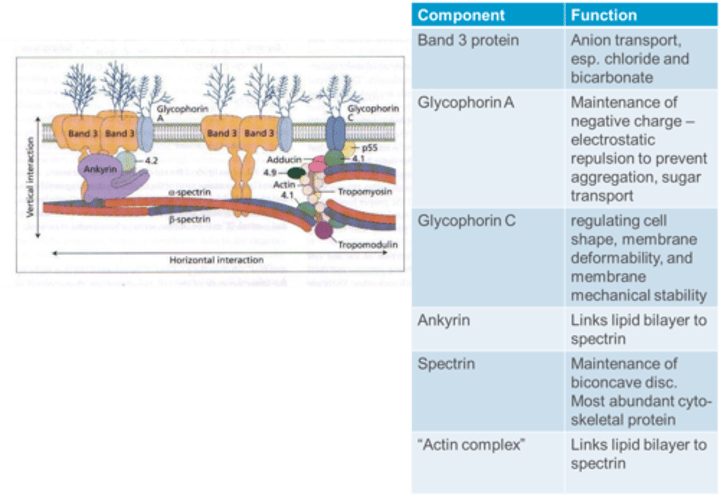
What is the function of Glycophorin A in the RBC membrane?
Glycophorin A contributes to the maintenance of a negative charge, providing electrostatic repulsion to prevent aggregation, and is involved in sugar transport.
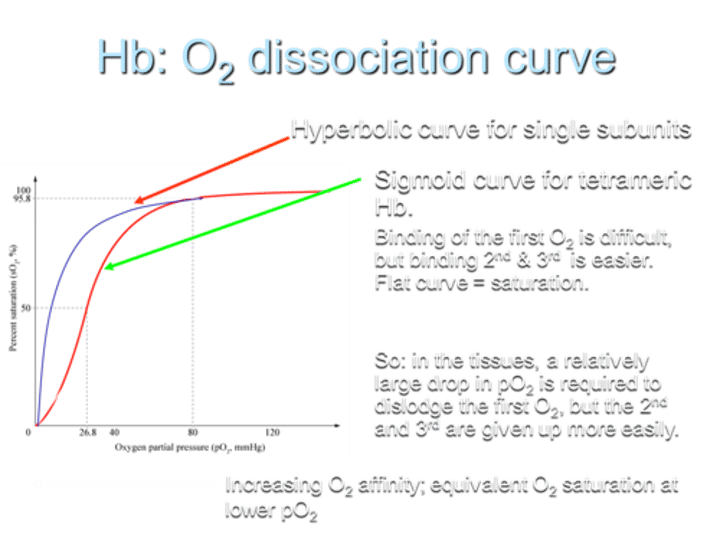
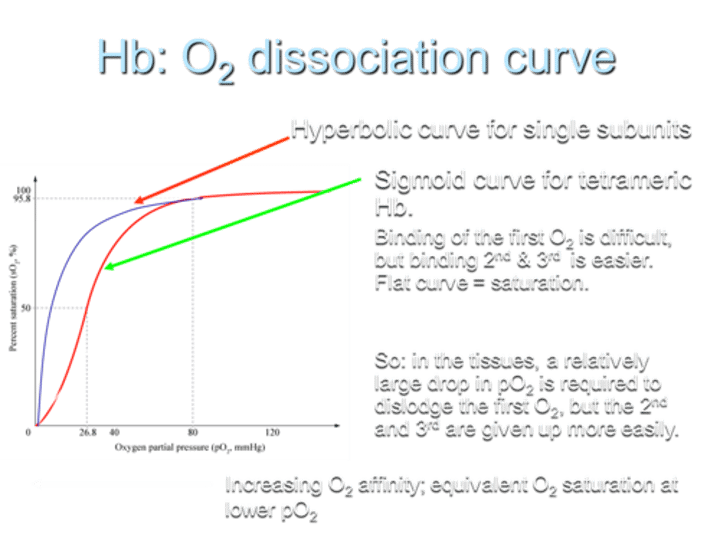
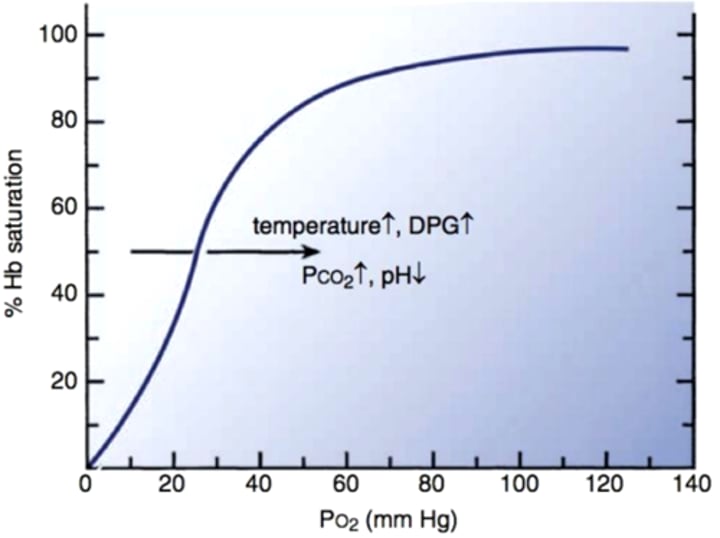
What type of O2 dissociation curve does Hemoglobin (Hb) exhibit for single subunits?
Hemoglobin shows a hyperbolic O2 dissociation curve for single subunits.
What is O2 affinity?
the relationship between Hb O2 saturation and pO2
(how strongly Hb is bonded to its oxygen at different pO2)
What type of O2 dissociation curve does Hemoglobin (Hb) exhibit for tetrameric Hb?
For tetrameric Hemoglobin (Hb), the O2 dissociation curve is sigmoid.
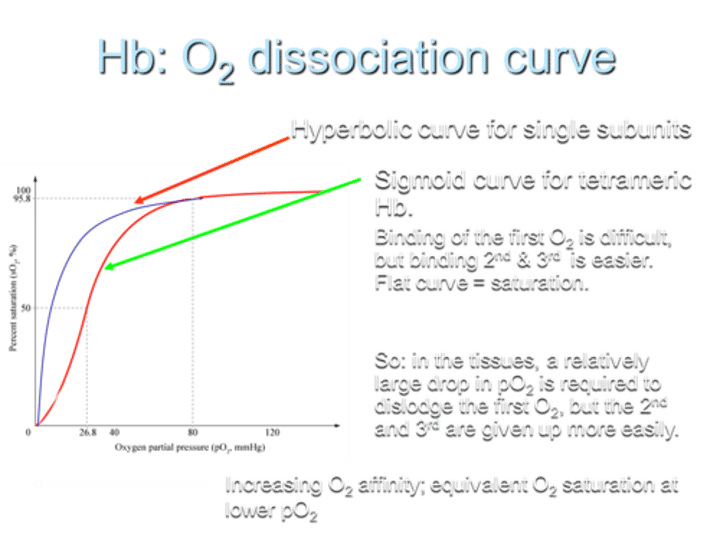
Describe the difficulty in O2 binding on the curve.
Binding of the first O2 is difficult, but binding the 2nd and 3rd O2 molecules is easier, resulting in a flat curve at saturation.
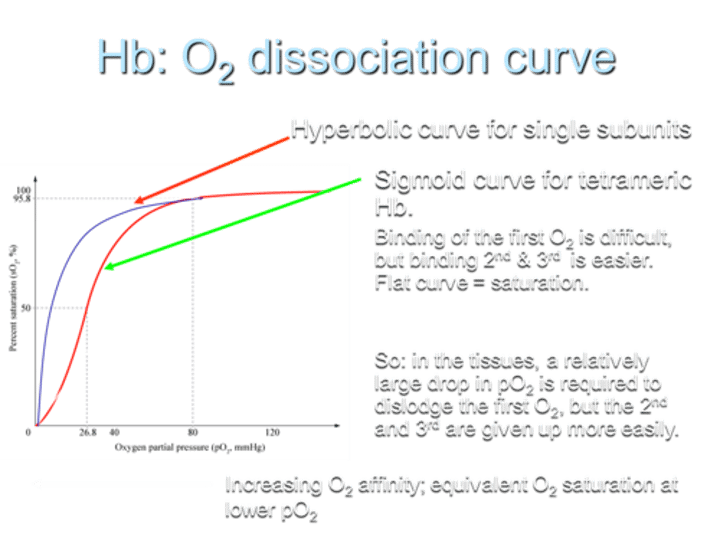
What does the sigmoid shape of the curve indicate?
The sigmoid shape indicates that in tissues, a relatively large drop in partial pressure of oxygen (pO2) is required to dislodge the first O2, while the 2nd and 3rd O2 molecules are given up more easily.
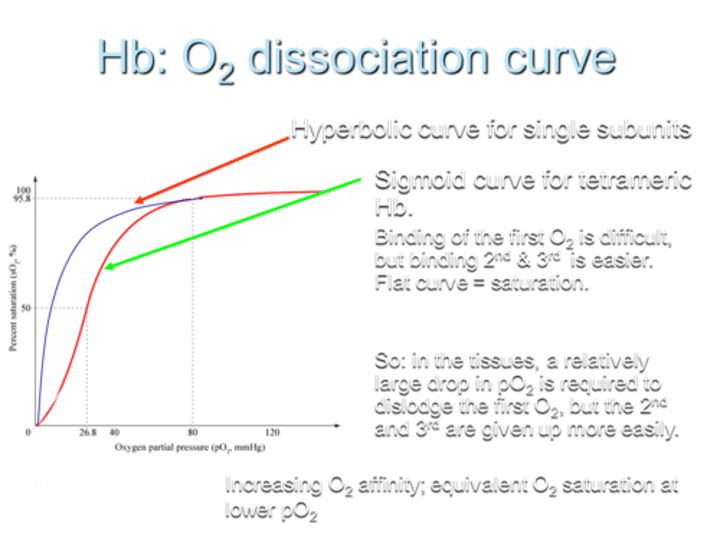
What is the result of increasing O2 affinity in terms of the curve?
Increasing O2 affinity results in equivalent O2 saturation at lower pO2.
Functional aspects of sigmoid curve
High affinity of fully oxygenated Hb prevents arterial deoxygenation
After the first O2 is given up, the next two are more easily released, improving delivery to metabolically active tissue
Conformational change - oxy / deoxy Hb pic
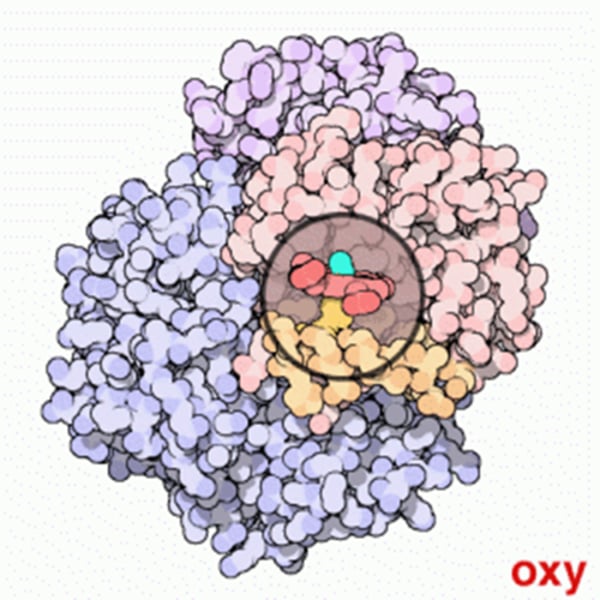
How can O2 affinity be varied in Hemoglobin (Hb)?
Conformational changes adjust the O2 affinity of tetrameric Hemoglobin (Hb) by varying O2 access to/from Haem groups.
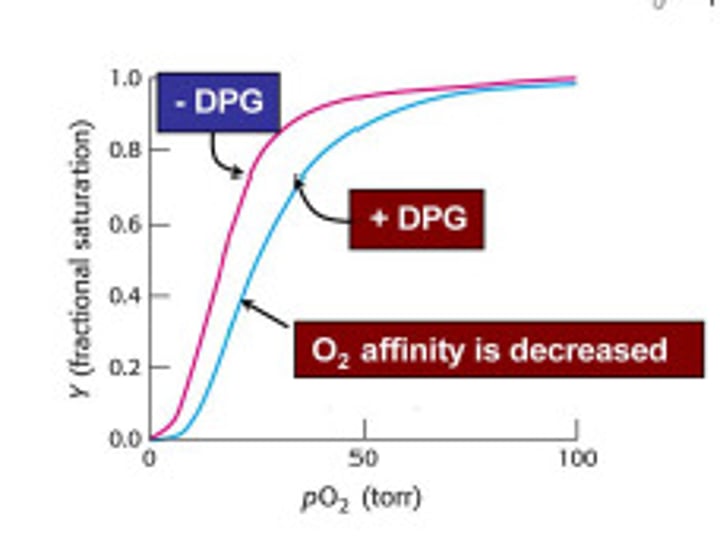
What metabolite preferentially binds to deoxygenated Hemoglobin, reducing its oxygen affinity further?
2,3-Diphosphoglycerate (2,3DPG or 2,3BPG), a metabolite, binds preferentially to deoxyHb, reducing its oxygen affinity further.
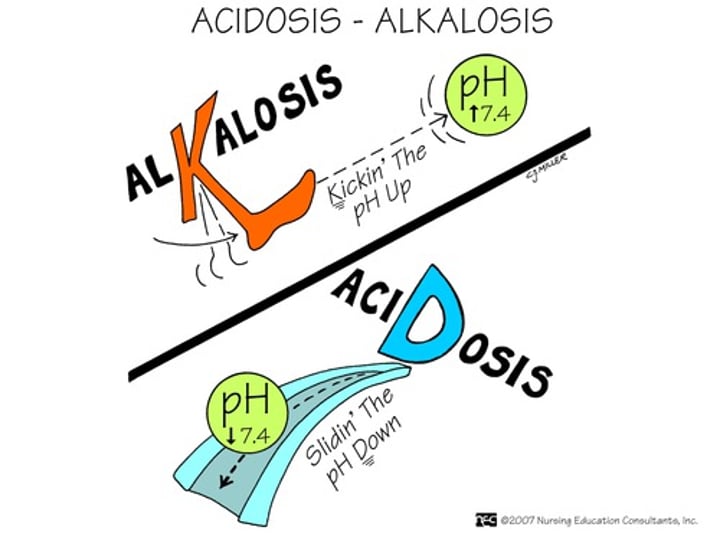
How does acidosis (blood pH <7.35) affect the O2 affinity of Hemoglobin?
Acidosis reduces O2 affinity (Bohr effect), increasing O2 supply.
Why does Hemoglobin F (HbF) have increased O2 affinity?
Hemoglobin F (HbF) has increased O2 affinity due to less active binding (by γ chains) to 2,3-DPG. This is essential for O2 supply to the developing fetus.
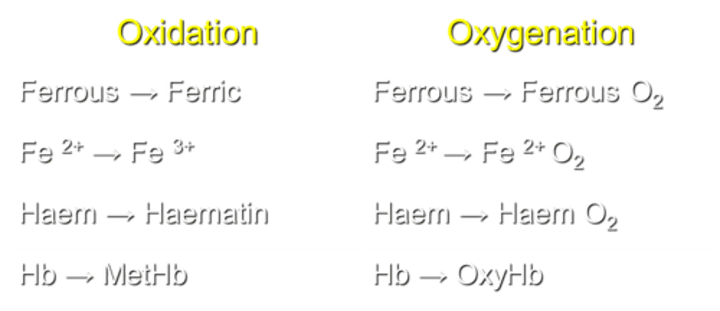
What is the process of converting ferrous to ferric in Hemoglobin (Hb)?
Oxidation transforms Fe2+ to Fe3+ in Hb.
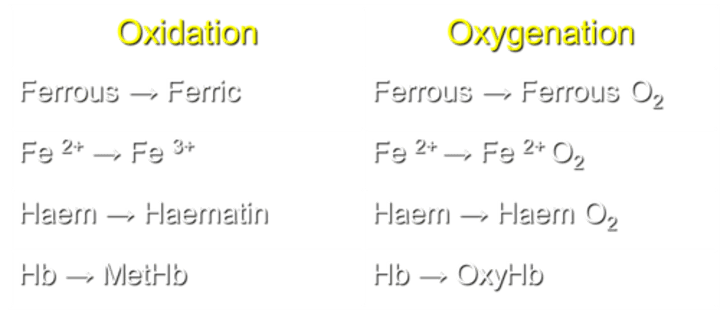
What occurs during the process of oxygenation in Hemoglobin (Hb)?
Oxygenation converts Fe2+ to Ferrous O2 in Hb.
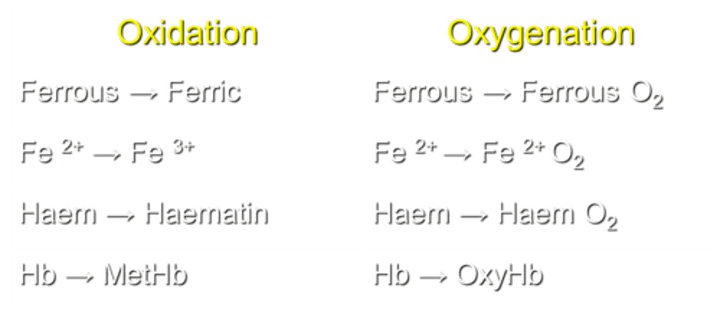
How is Haem transformed during the oxidation process?
Haem is converted to Haematin during oxidation.
What transformation occurs in Haem during the process of oxygenation?
Haem transforms into Haem O2 during oxygenation.
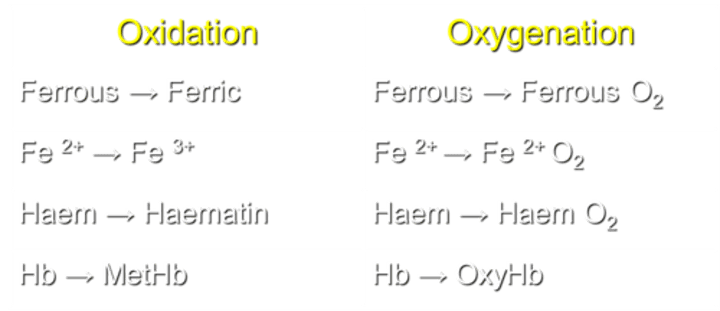
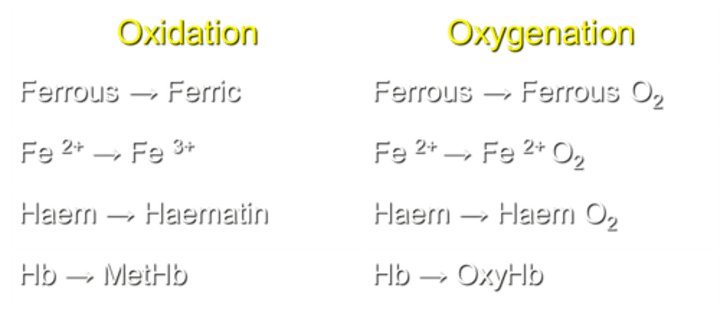
What is the result of Hemoglobin oxidation?
Hb transforms into MetHb during oxidation.
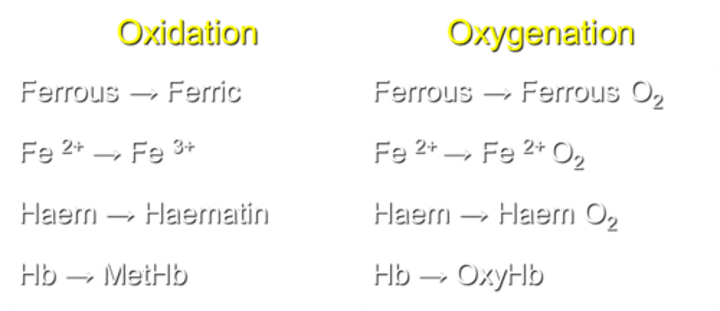
What is the result of Hemoglobin oxygenation?
Hb transforms into OxyHb during oxygenation.
What are potential advantages of artificial red blood cells over transfused human RBCs?
Storage temperature and shelf-life
Immediate, universal administration
No lag in effectiveness as with natural blood (due to 2,3-DPG and nitric oxide depletion during storage)
No risk of disease transmission
Not dependant on donors
Avoid religious / cultural issues, e.g. Jehovah's Witnesses
Why can't free globin chains be transfused in solution as an artificial red blood cell?
Free globin chains are toxic to kidneys and scavenge nitrous oxide, leading to vasoconstriction and hypertension.
What are the drawbacks of transfusing hemoglobin in solution?
Unfavorable fixed O2 affinity.
Short half-life (30 minutes).
What is an example of "re-manufactured" Hb used as an artificial red blood cell?
Examples include polymerized bovine globin chains, such as Hemopure (licensed in South Africa in 2001).
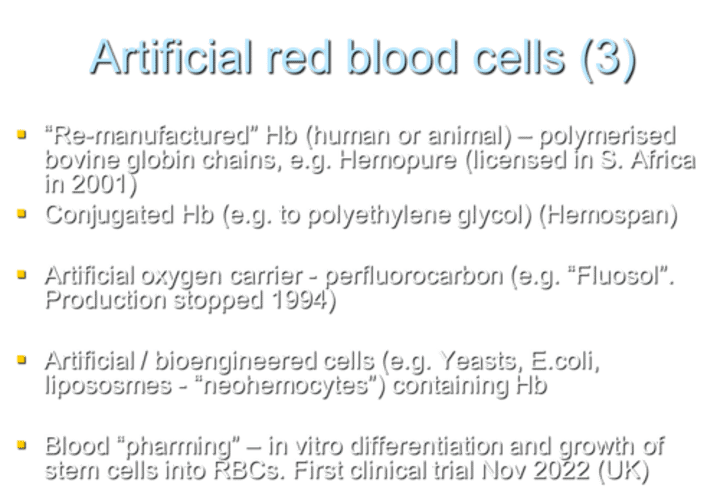
What is an example of conjugated Hb used as an artificial red blood cell?
Hemospan is an example of conjugated Hb, where Hb is conjugated to polyethylene glycol.

What is an example of an artificial oxygen carrier that was produced but discontinued in 1994?
Fluosol is an example of an artificial oxygen carrier using perfluorocarbon. Production was stopped in 1994.
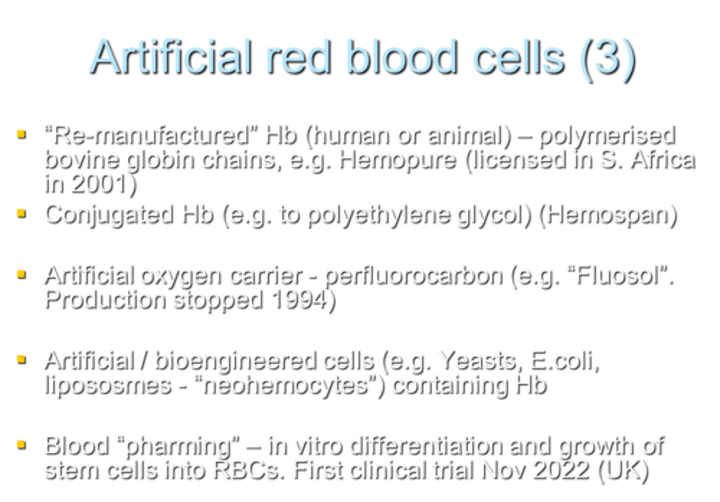
What are examples of artificial/bioengineered cells containing Hb?
Examples include cells derived from yeasts, E. coli, and liposomes, often referred to as "neohemocytes."
What is "blood pharming" in the context of artificial red blood cells?
Blood pharming involves in vitro differentiation and growth of stem cells into RBCs. The first clinical trial for this approach started in November 2022 in the UK.
Anaemia =
reduced [Hb] in blood
![<p>reduced [Hb] in blood</p>](https://knowt-user-attachments.s3.amazonaws.com/4f465e63-11b2-4be2-b881-8a206ed9034d.jpg)
At what hemoglobin concentration ([Hb]) do symptoms of anemia generally appear?
Symptoms of anemia generally appear when hemoglobin concentration ([Hb]) falls below 90 - 100 g/l.
![<p>Symptoms of anemia generally appear when hemoglobin concentration ([Hb]) falls below 90 - 100 g/l.</p>](https://knowt-user-attachments.s3.amazonaws.com/9ffb2c09-27f5-47c5-a32d-8c7c4cfbf44f.jpg)
How is slow-onset anemia tolerated compared to rapid-onset anemia?
Slow-onset anemia is much better tolerated than rapid-onset anemia. Symptoms may appear at levels as low as 60 g/l in an otherwise well, young patient.

What are common symptoms of anemia?
• Shortness of breath
• Weakness
• Pallor
• Lethargy
• Palpitations
• Headaches
• Heart failure and confusion (older patients)
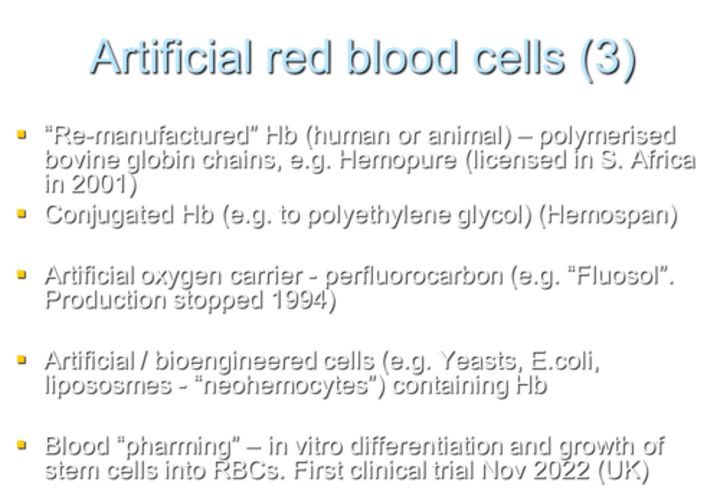
What is a clinical sign of anemia related to mucous membranes and nail beds?
Pallor of mucous membranes and nail beds is a clinical sign of anemia.
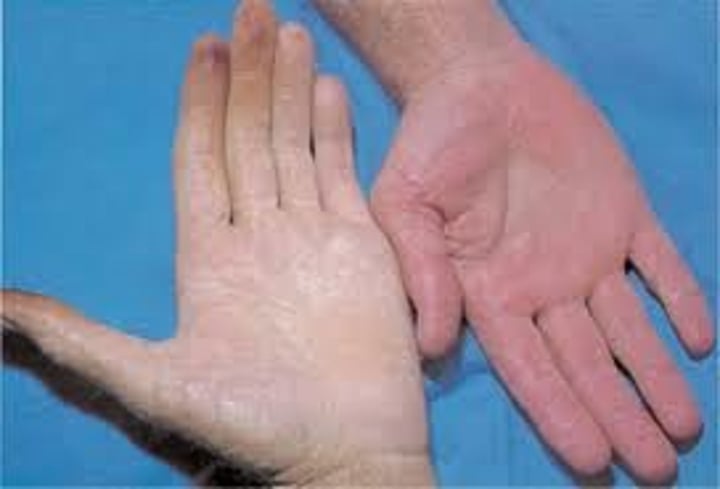
What nail abnormality may be associated with certain types of anemia?
Concave nails (koilonychia) may be associated with certain types of anemia.
Name a clinical sign of anemia that involves yellowing of the skin.
Jaundice is a clinical sign of anemia involving yellowing of the skin.
What are some potential signs of anemia depending on its type?
• Leg ulcers
• Bone deformities
• Recurrent infection and / or bruising if due to e.g.
bone marrow failure or leukaemia.
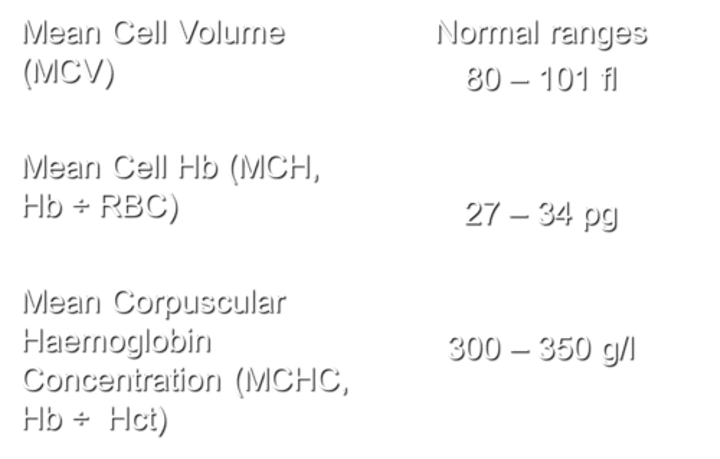
What does MCV stand for, and what are the normal ranges?
MCV stands for Mean Cell Volume. The normal ranges for MCV are 80-101 fl.
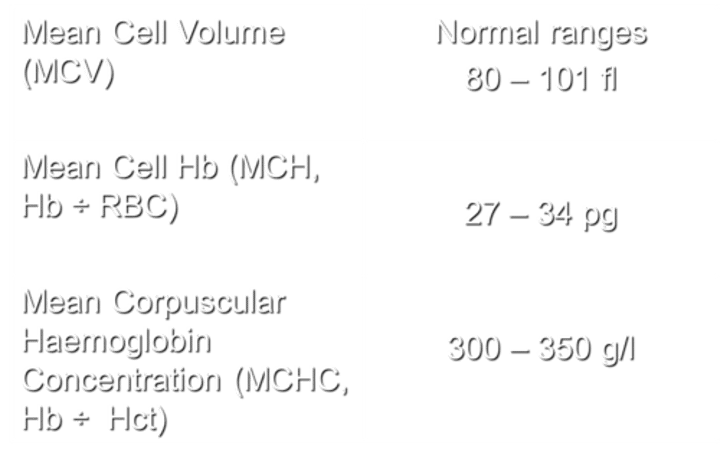
What does MCH stand for, and how is it calculated?
MCH stands for Mean Cell Haemoglobin. It is calculated by dividing the haemoglobin (Hb) by the number of red blood cells (RBC).
with normal ranges being 27 - 34 pg.
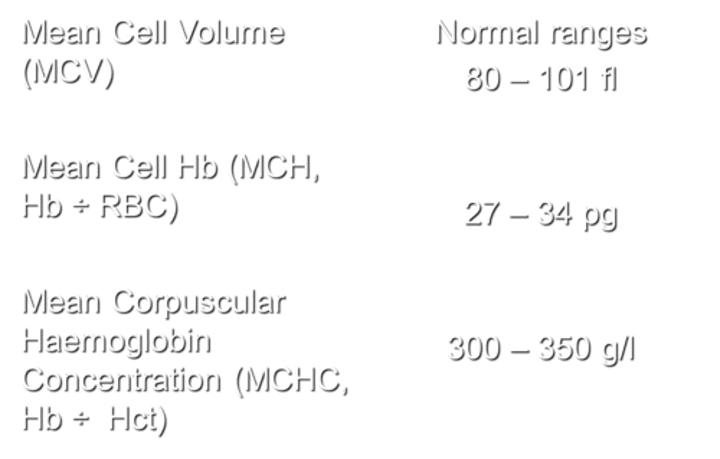
What does MCHC stand for, and how is it calculated?
MCHC stands for Mean Corpuscular Hemoglobin Concentration. It is calculated by dividing the hemoglobin (Hb) by the hematocrit (Hct), with normal ranges being 300-350 g/l.
How is anemia usually classified based on two RBC parameters?
Anemia is usually classified based on whether the RBCs are:
Small (microcytic)
Normal size (normocytic)
Large (macrocytic)
What is the second classification based on RBC parameters?
Anemia can also be classified based on whether the RBCs have:
Reduced hemoglobin concentration (hypochromic)
Normal hemoglobin concentration (normochromic)
High hemoglobin concentration (hyperchromic, limited to spherocytosis (inherited blood disorder) only)
Besides size and hemoglobin concentration, how else can anemia be classified?
But can also classify by
impaired production (e.g. Fe, vitamin B12 / folate deficiency, some haemoglobinopathies (inherited disorders of globin))
or
increased RBC loss / destruction (bleeding / haemolytic anaemias, some haemoglobinopathies)
What characterizes normocytic, normochromic anemia?
Normocytic, normochromic anemia is characterized by:
Normal volume of RBCs (MCV is normal)
Normal amount of hemoglobin (MCH is normal)
Normal concentration of hemoglobin (MCHC is normal)Reduced RBC count
Can you provide examples of conditions leading to normocytic, normochromic anemia?
Conditions leading to normocytic, normochromic anemia include:
Acute bleed
Marrow failure
Hemolysis
Renal failure (Epo deficiency)
What characterizes microcytic, hypochromic anemia?
Microcytic, hypochromic anemia is characterized by:
Reduced volume of RBCs (MCV is reduced)
Less hemoglobin at a lower concentration than normal (MCH and MCHC are reduced)
Can you provide examples of conditions leading to microcytic, hypochromic anemia?
Conditions leading to microcytic, hypochromic anemia include:
Iron deficiency (absolute or functional)
Thalassemia (inherited conditions effecting haemoglobin)
Anemia of chronic disorders
What characterizes macrocytic anaemia
Macrocytic anemia is characterized by:Low RBC countIncreased volume of RBCs (MCV is increased)Hemoglobin at a normal concentration (MCHC is normal)Increased MCH
Can you provide examples of conditions leading to macrocytic anaemia?
Conditions leading to macrocytic anemia include:
B12 deficiency
Folate deficiency
What are examples of conditions leading to anemia due to bleeding?
Examples include acute and/or chronic bleeding.
What are examples of conditions leading to anemia due to haematinic deficiency?
Examples include deficiencies in iron (Fe), vitamin B12, or folate.