7-Alkenes and Alkynes
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
atoms that get attacked by other atoms containing an abundance of e-
electrophiles
atoms that attack other atoms with their electrons
nucleophiles
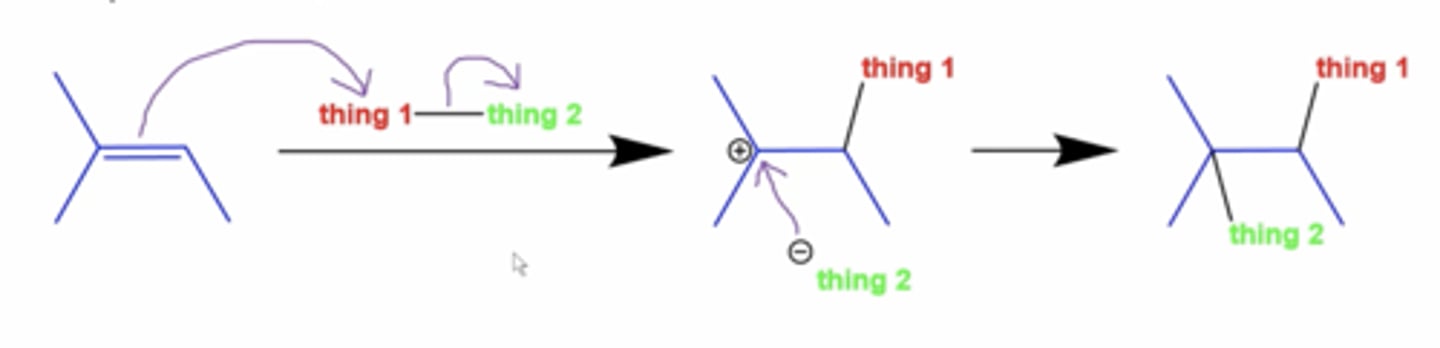
what is the general appearance of alkene addition reactions?
either side of the alkene gets shit added to it
-the thing that gets attacked goes on the carbon with more H's, and thing 2 goes on the carbon with fewer H's
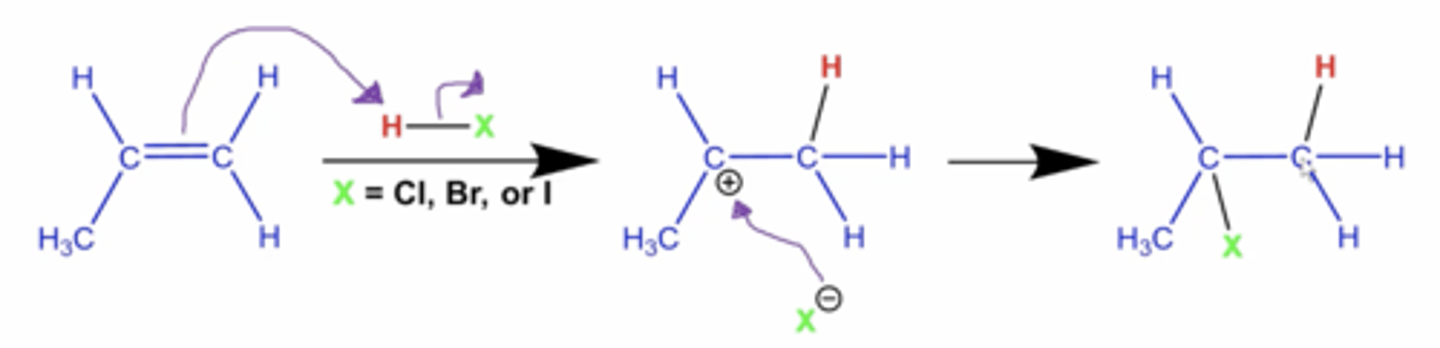
what is the general mechanism for hydrohalogenation reactions?
-halogen always goes on the carbon with fewer H's and the hydrogen always goes on the carbon with more H's on it
-the H goes on the carbon with more H's because secondary carbocation intermediates are more stable
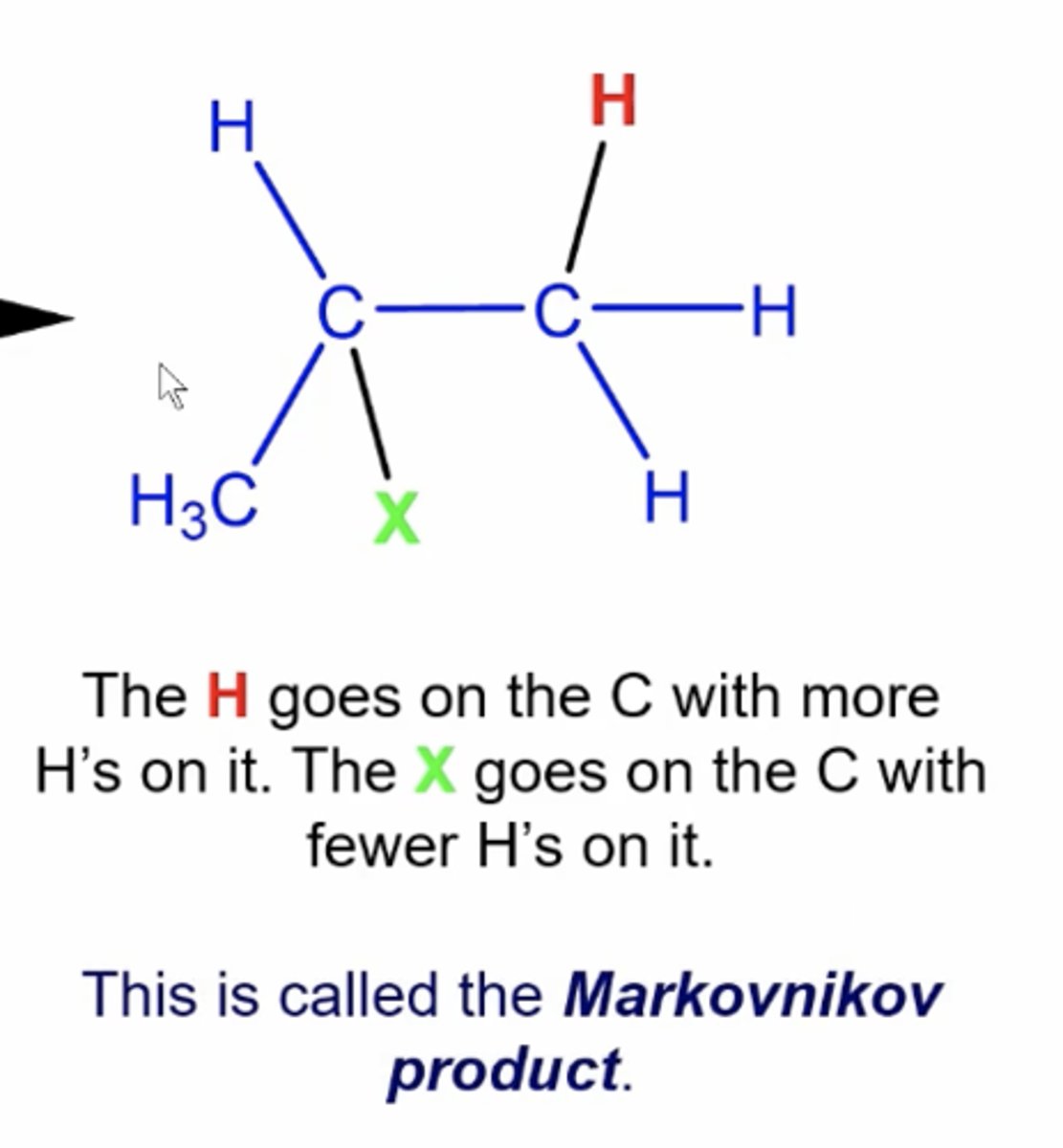
the product that is the alkene addition product that comes from the most stable carbocation intermediate
Markovnikov product
explain 1,2 hydride shifts
if you have a terminal alkene, a NEIGHBORING hydrogen can shift with the carbocation to form a more stable carbocation intermediate
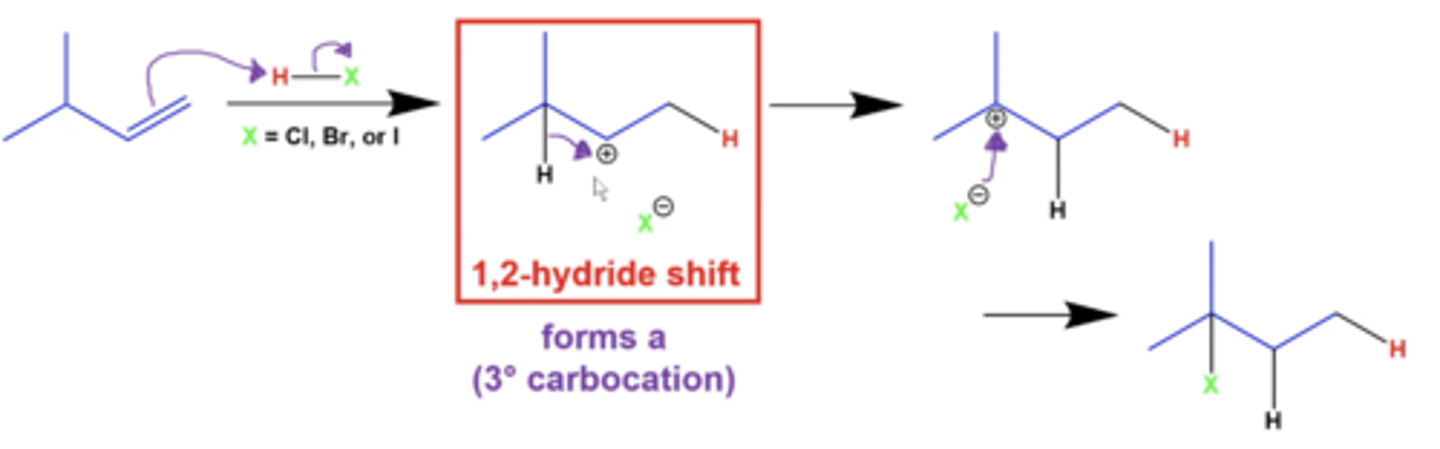
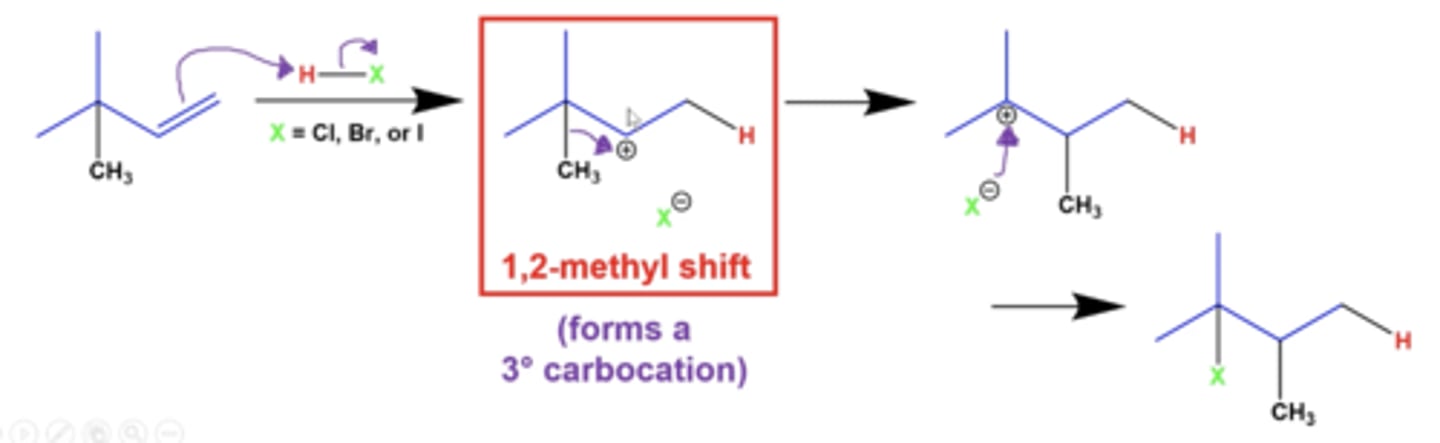
explain 1,2 methyl shifts
if you have a terminal alkene, a NEIGHBORING methyl group can shift with the carbocation to form a more stable carbocation intermediate
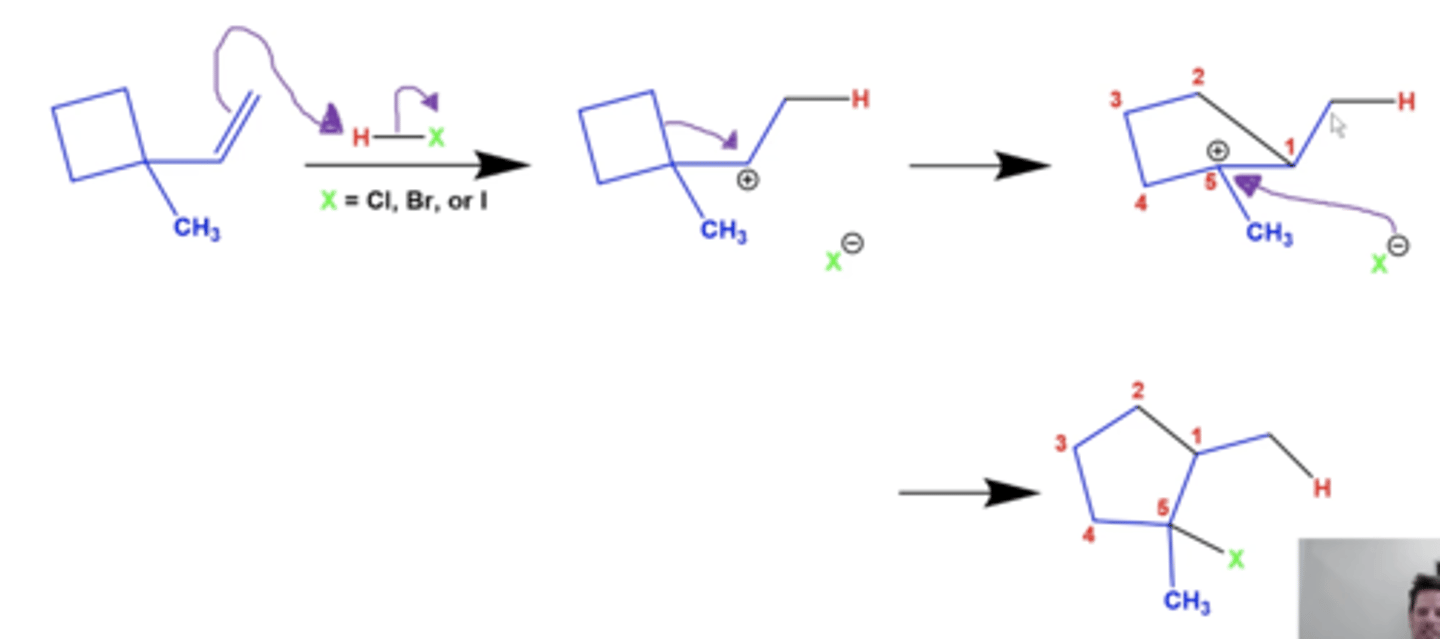
why do carbocation ring expansion rearrangements happen?
-they're usually most favorable when they involve forming rings with 5-6 carbons, in order to relieve angle strain
-five or six-membered rings are typically the most stable ring sizes, because other ring sizes force carbons into bond angles that deviate significantly from the ideal tetrahedral 109.5 degrees (angle strain)
what is the general mechanism for carbocation ring expansion rearrangements?
-this not only forms a more stable carbocation, but also relieves angle strain
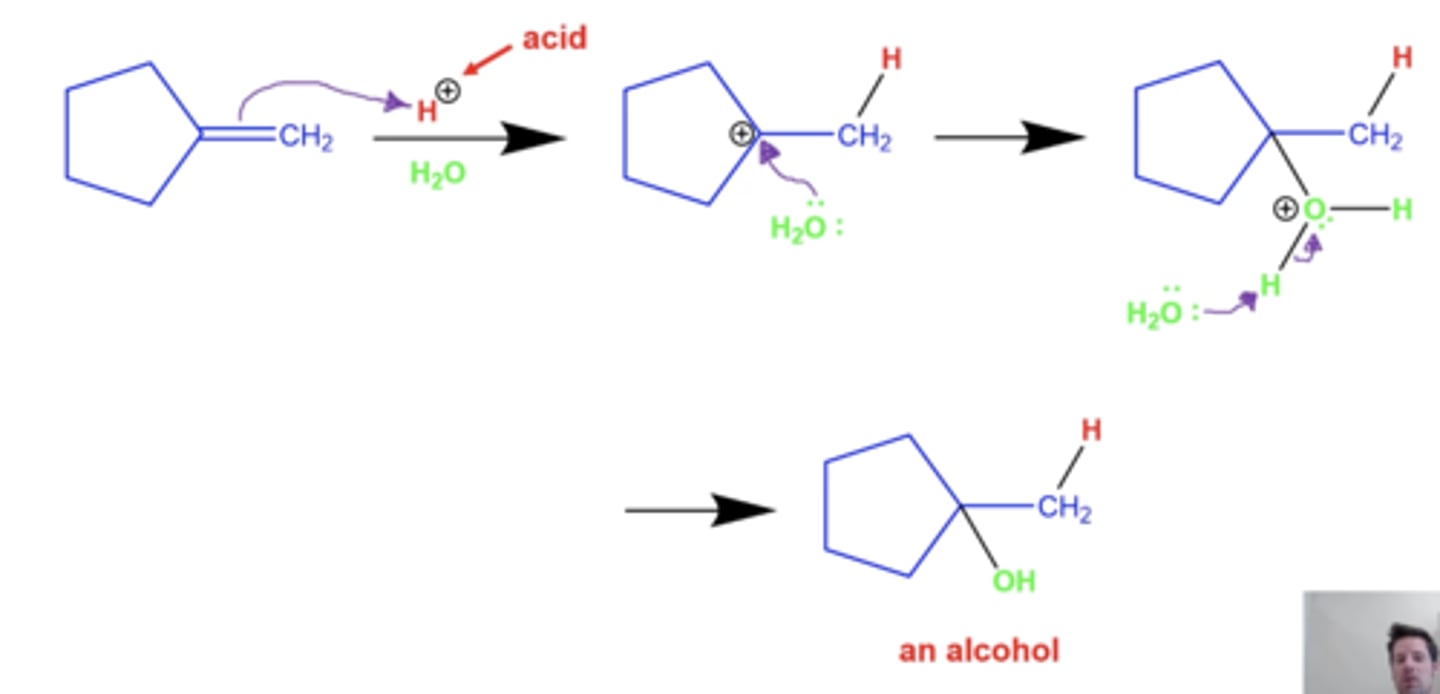
what is the general mechanism for acid-catalyzed hydration (adding OH to alkenes)?
these intermediates CAN undergo rearrangements if it makes a more stable carbocation
what is a reaction you can perform in order to prevent carbocation rearrangements unlike, acid-catalyzed hydration?
Oxymercuration-demercuration:
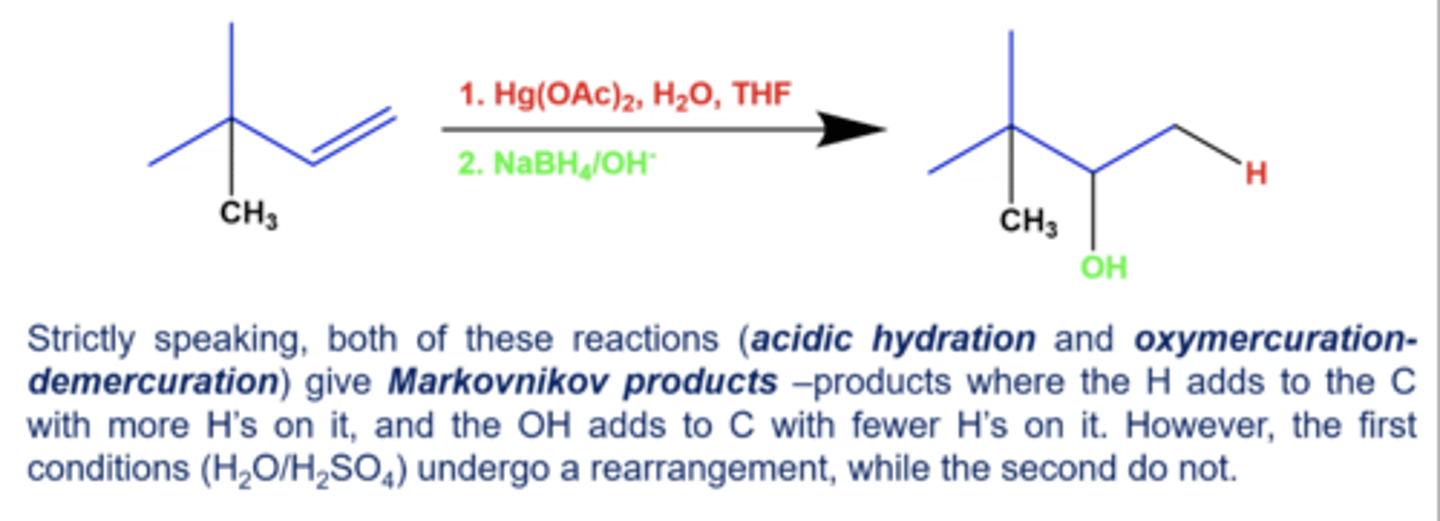
what is the summary for oxymercuration-demercuration?
what's added: H+ and OH-
regioselectivity: markovnikov (technically)
Intermediate: Mercurinium ion bridge
rearrangement: not possible
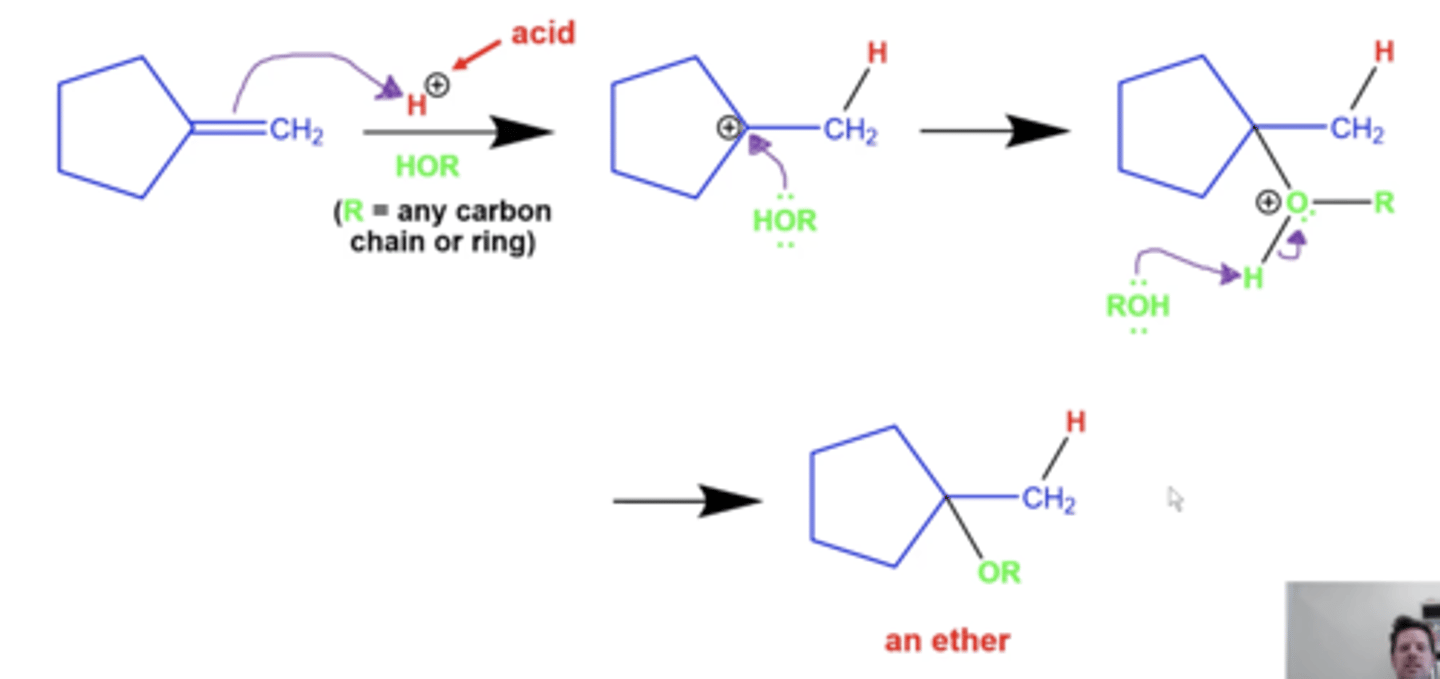
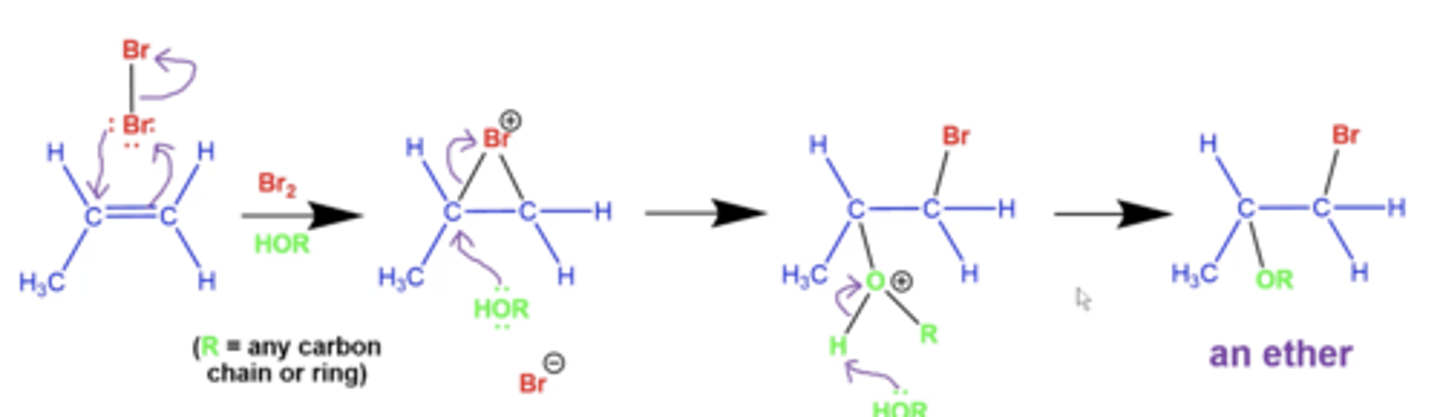
what is the general mechanism for acid-catalyzed alcohol addition (adding O-R to alkenes)?
this reaction produces an ether
rearrangements are ALSO possible here
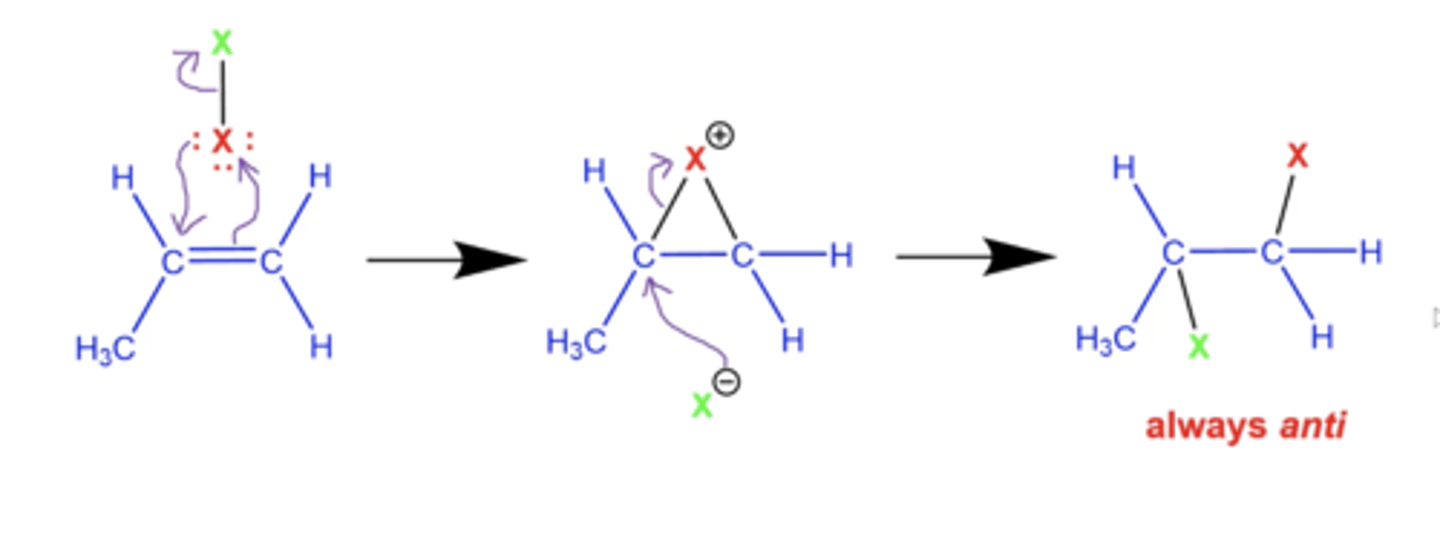
What is the general mechanism for adding halogens (X2) to alkenes?
-forms a very triangular "bromonium" ring
-one halogen inserts into either end of the double bond
-final product is always anti
-no rearrangements!
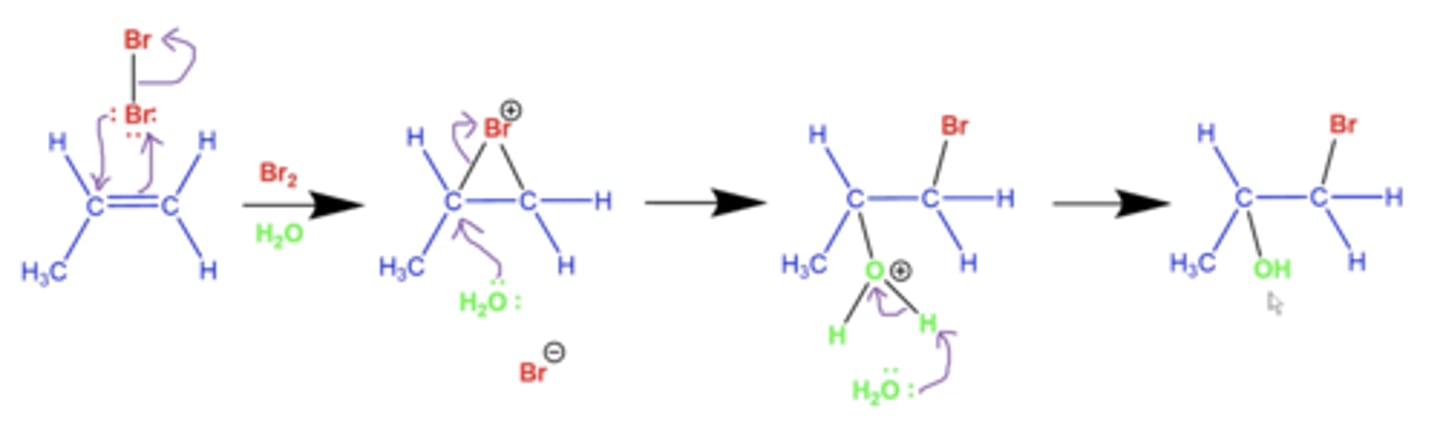
what is the general mechanism for adding X2 and H2O as solvent to alkenes?
OH attacks at the "bromonium bridge" instead of another halogen
-OH added to more substituted side
what is the general mechanism for adding X2 and ROH (alcohols) as solvent to alkenes?
-exactly the same mechanism as adding X2 and H2O, except you form an ether as a result
-product always anti
-OR added to more substituted side
what are anti-markovnikov reactions?
these are products in which OH adds to the LESS substituted carbon of an alkene, and H to the MORE substituted carbon of an alkene
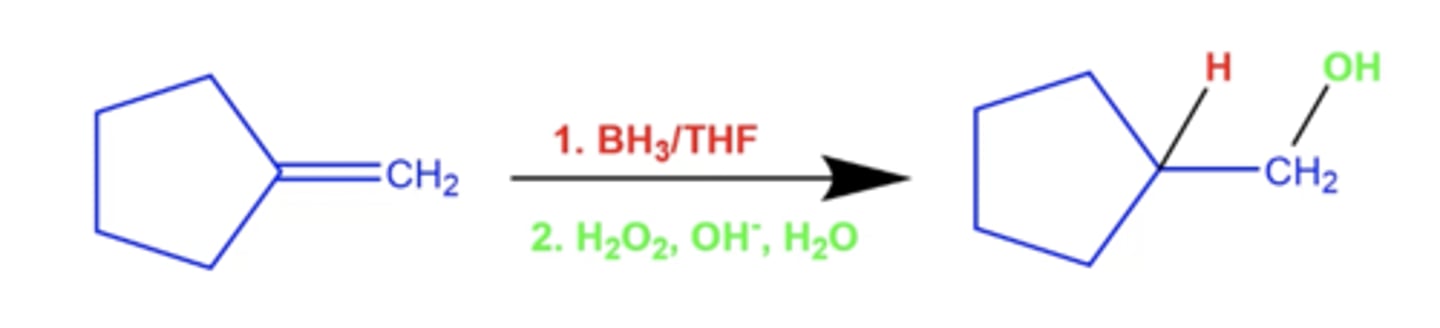
what reactions can produce anti-markovnikov products with H and OH?
what solvents do these use?
hydroboration-oxidation reactions with these solvents:
-the H and OH end up syn (cis) to each other
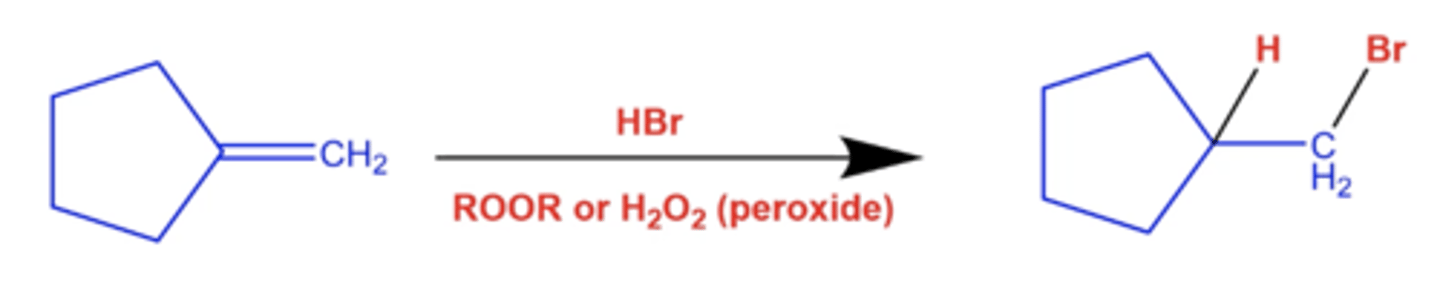
how can you form the anti-markovnikov product for reactions with Br?
use hydrobromination with peroxide (ROOR):
add HBr and peroxide, which adds Br to the less substituted side and H to the more substituted side
-this does not cause rearrangements!
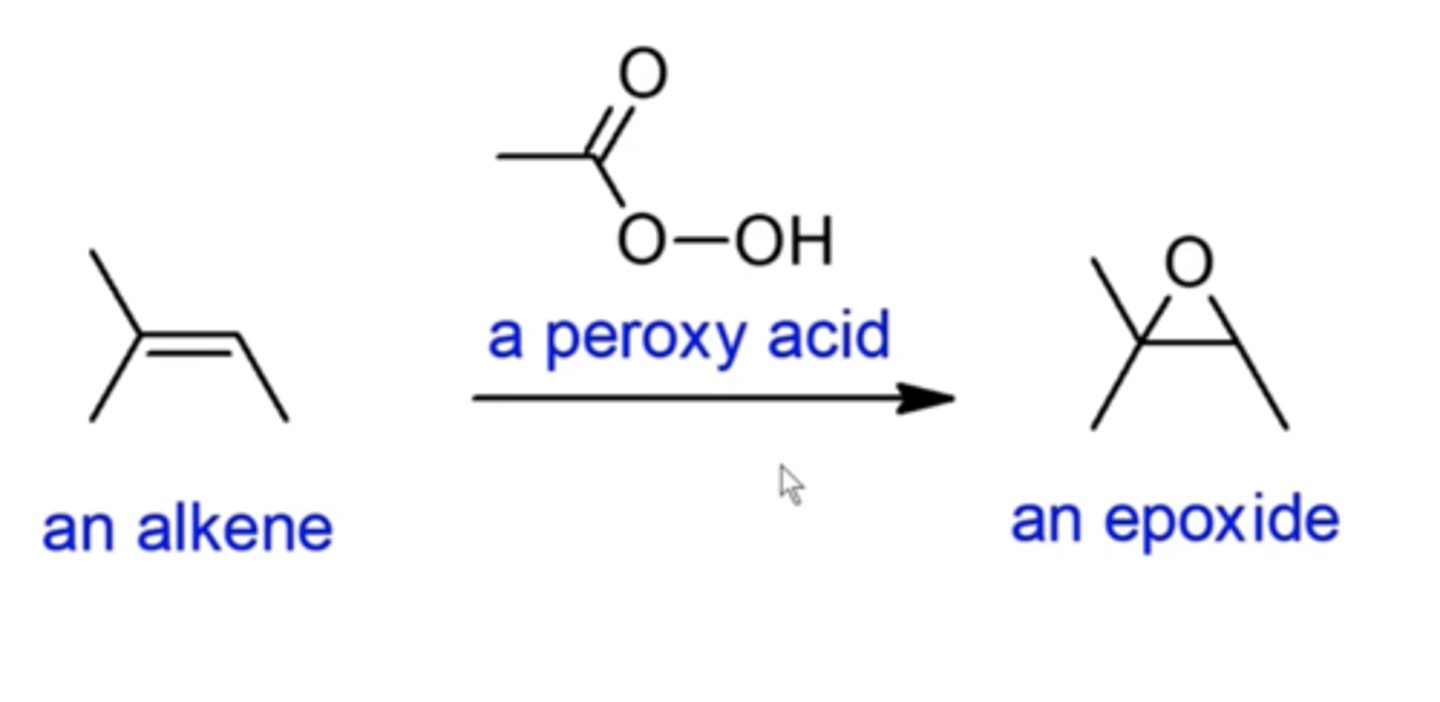
what happens if you add a peroxy acid (looks like a C.A., but with another OH attached to the O) to an alkene?
epoxidizing alkene reactions:
it forms an epoxide
what is a major peroxy acid to use if you want to do an epoxidizing reaction with an alkene?
mCPBA
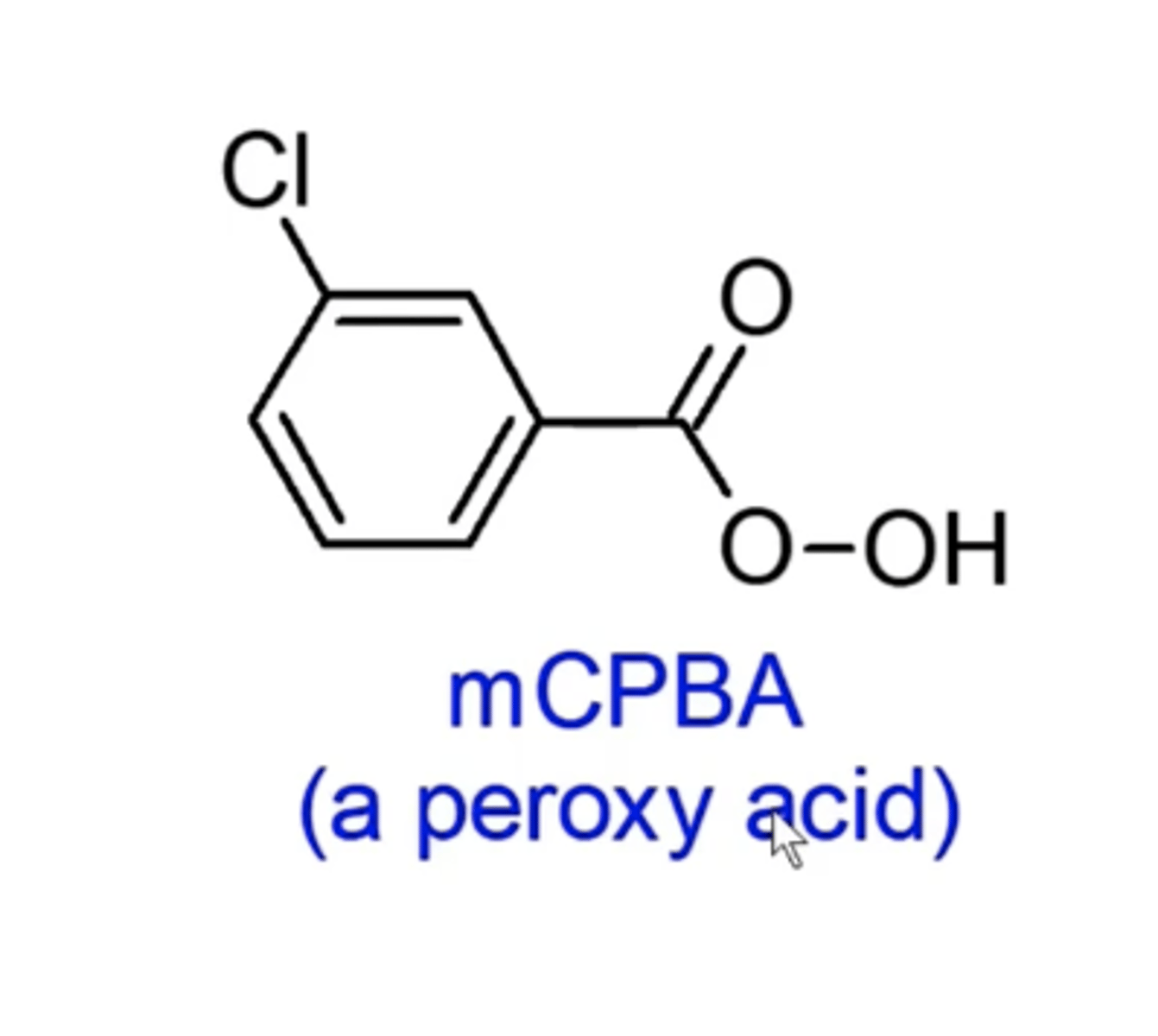
what happens if you add nucleophiles to epoxides under basic conditions?
-nucleophile will attack at the less substituted carbon of the epoxide
-you can then add H+ in a workup to make OH
this approach forms di-hydroxides that are anti
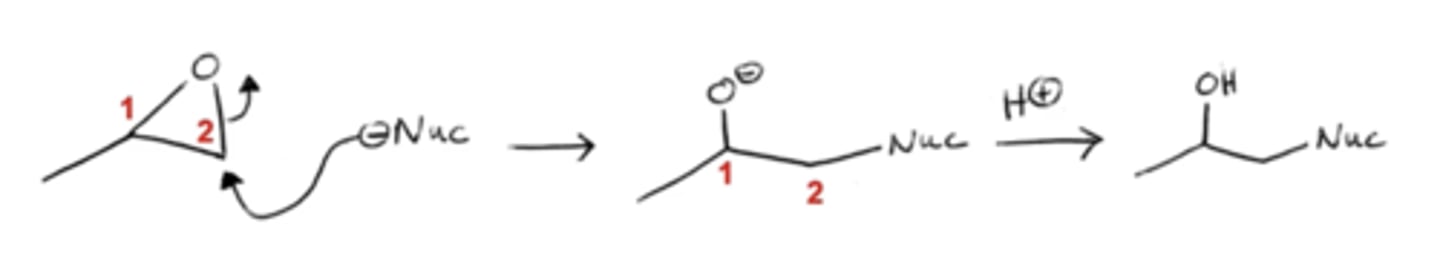
what happens if you add nucleophiles to epoxides under acidic conditions?
-the oxygen of the epoxide will attack a free-floating hydrogen, creating an OH group
-the nucleophile will attack the more substituted carbon to open the epoxide ring
this approach forms di-hydroxides that are anti
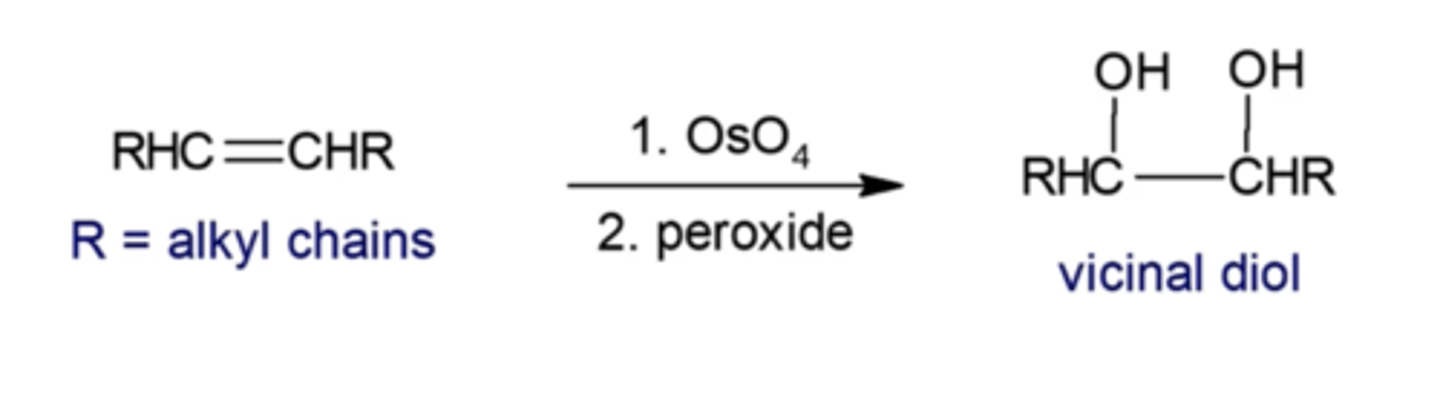
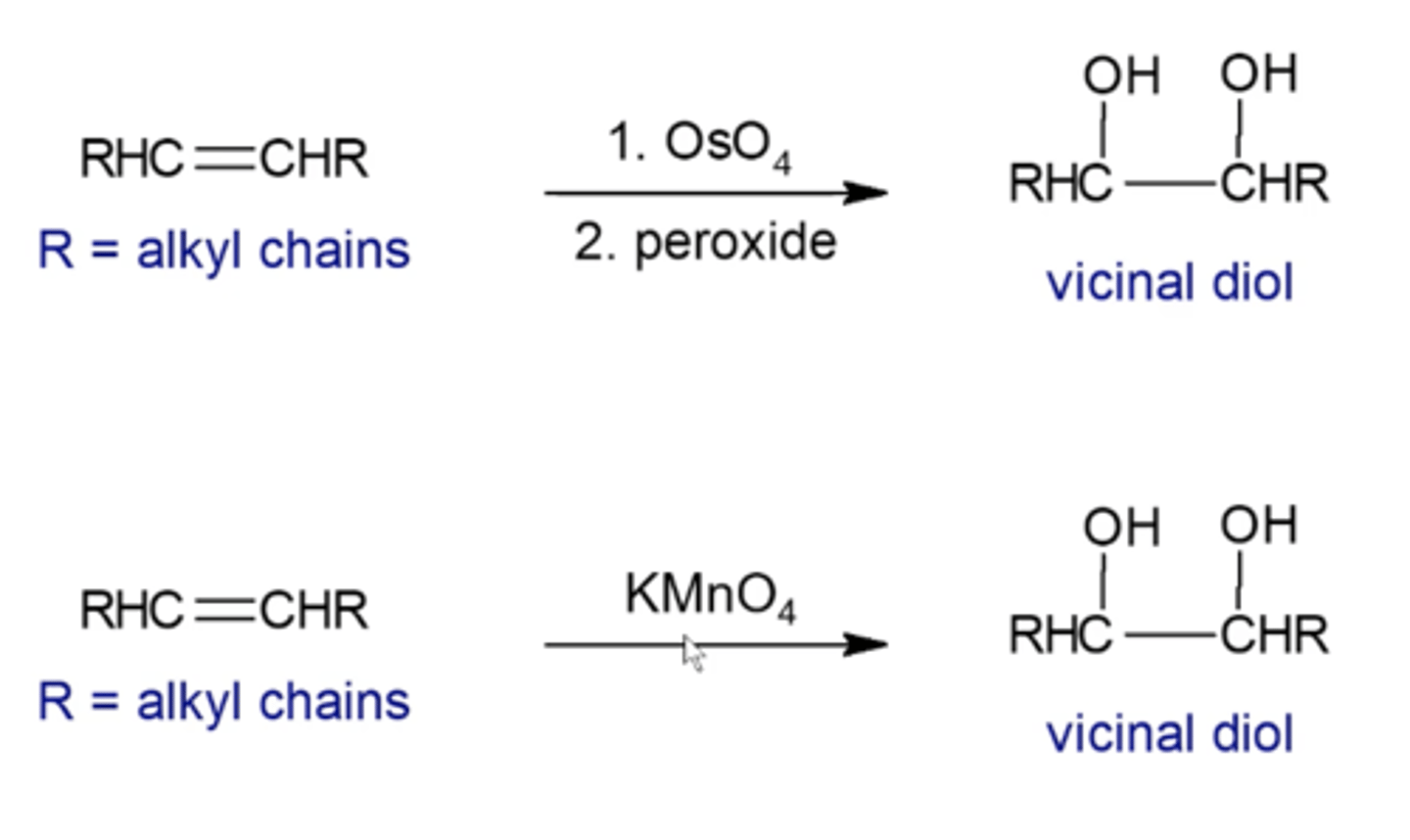
how do you perform a syn-dihydroxylation?
you must use OsO4 followed by H2O2
-OH's always end up syn to each other
what happens if you treat an alkene with ozone (O3; ozonolysis)?
it "saws" the double bond in half and places an oxygen at the end of each half
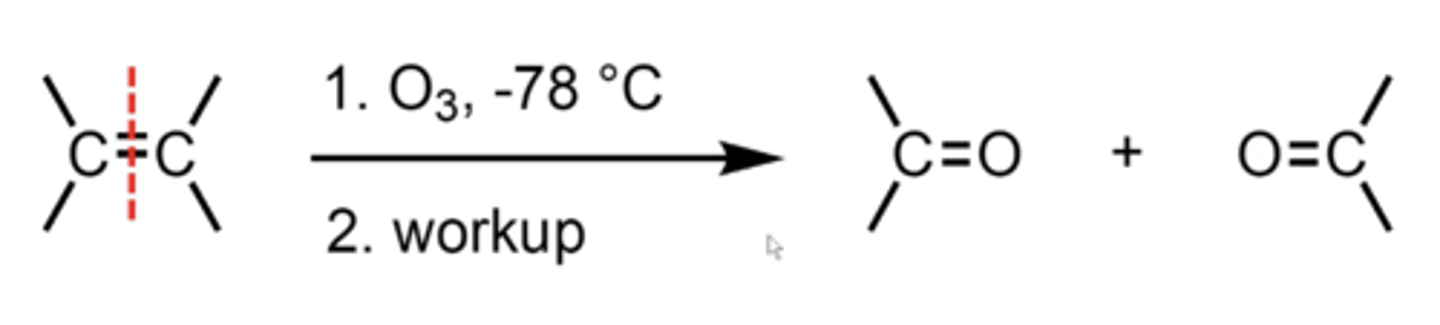
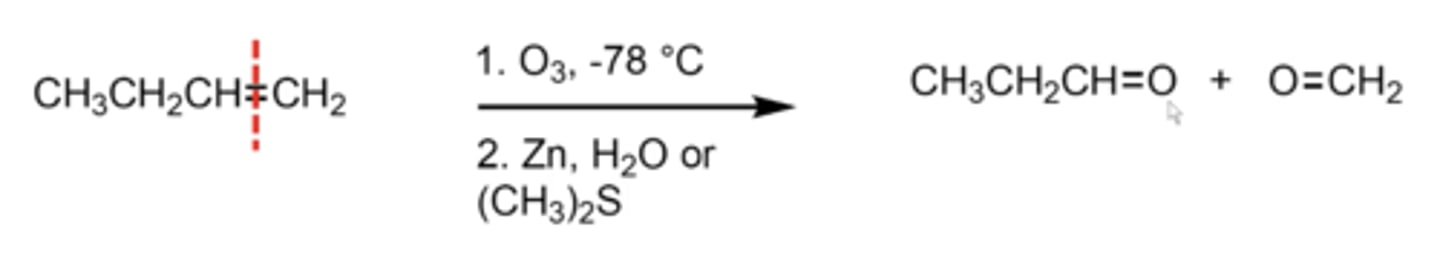
what happens if you perform ozonolysis with a Zn/H2O or DMS ((CH3)2S) workup?
just add one oxygen to each of the alkene carbons
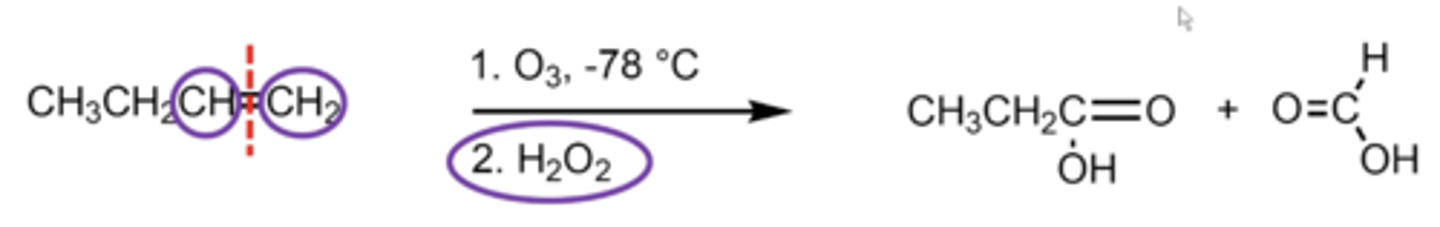
what happens if you perform ozonolysis with a H2O2 (hydrogen peroxide) workup?
if either of the alkene carbons have at least one hydrogen attached, you replace one of the alkene hydrogens with an OH on either side, producing C.A. products
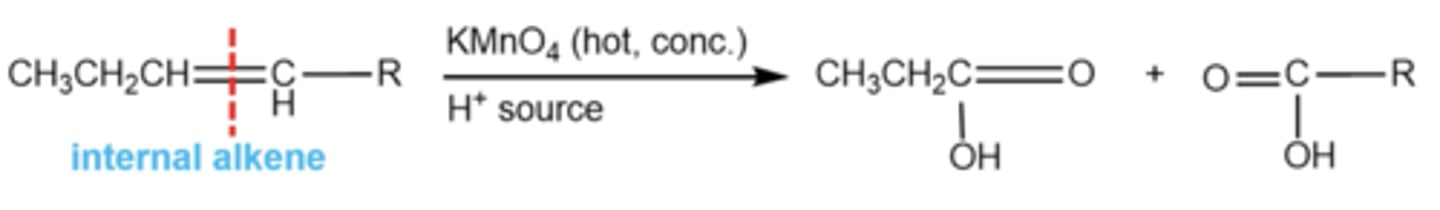
what happens if you have an internal alkene with hot conc KMnO4/H3O+?
it does the exact same thing as O3 and H2O2: ozonolysis
what happens if you have an terminal alkene with hot conc KMnO4/H3O+?
the same ozonolysis will occur, except the terminal carbon will be converted into CO2, because all connected hydrogens on the terminal end get converted to O's
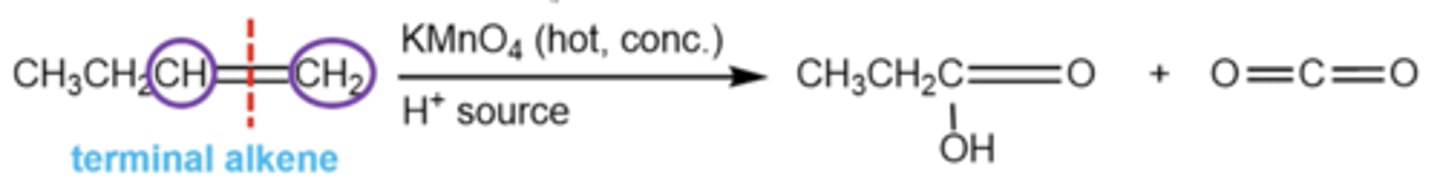
how can you saw alkenes in half to convert them into aldehydes?
first, use OsO4 with a quench
then, add HIO4, which saws the molecule between the two alcohols, turning them into aldehydes
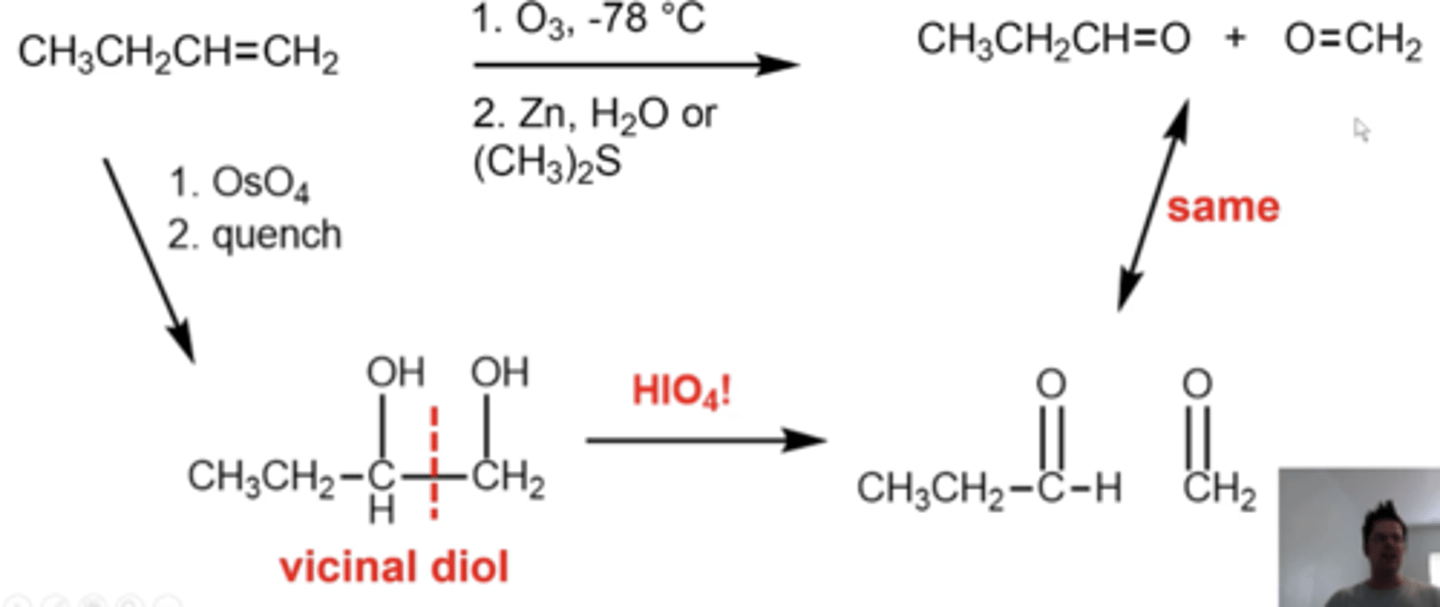
what happens if you add KMnO4 without acid added?
it will do the same thing as OsO4 and peroxide (you'll convert an alkene into a vicinal diol)
what happens if you treat an alkyne with ozone (O3)?
it also "saws" the triple bond in half and converted each former alkyne carbon into a C.A. carbon
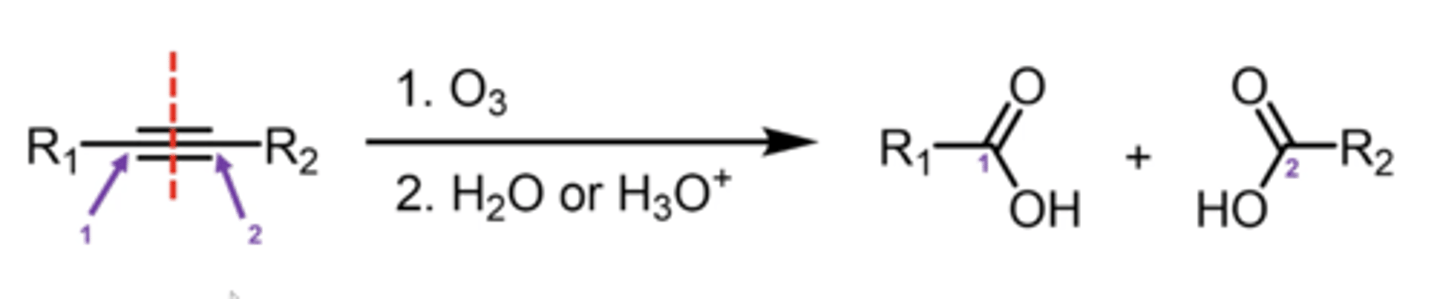
what happens if you treat an alkyne with basic KMnO4 and H3O+?
the same thing as ozone (O3):
it also "saws" the triple bond in half and converted each former alkyne carbon into a C.A. carbon
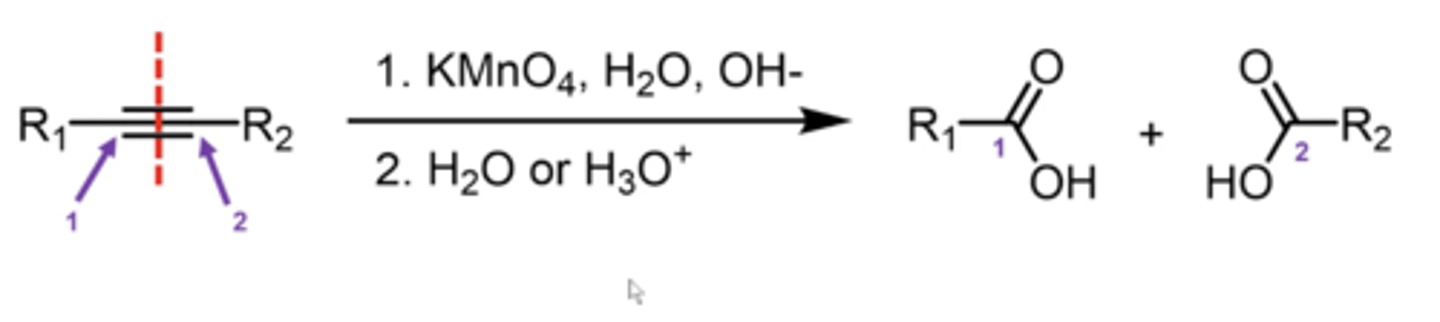
what happens if you're performing ozonolysis on a terminal alkyne?
it forms a C.A. and CO2
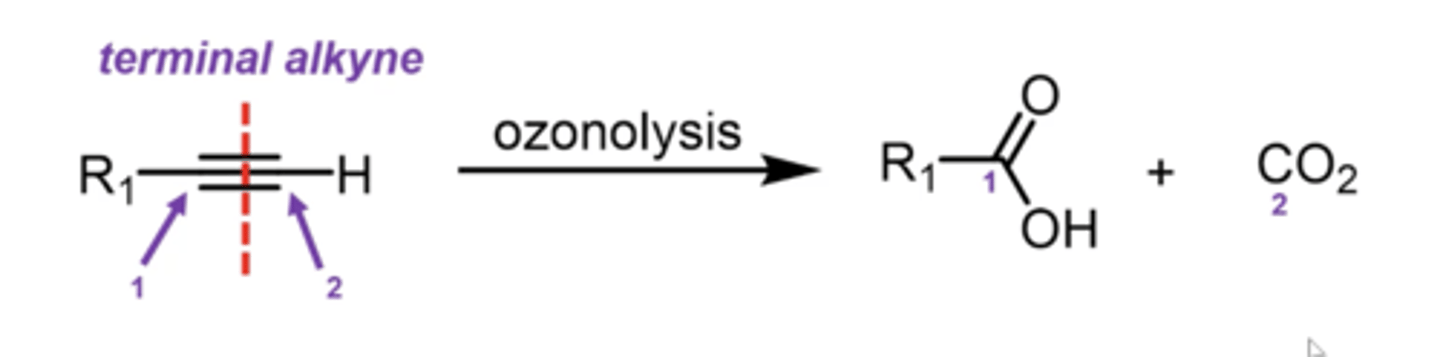
what happens if you take an alkene and treat it with H2 plus Pd, Pt, Rh, or Ni as a catalyst?
you perform catalytic hydrogenation of alkenes:
alkenes get converted into alkanes, with 2 hydrogens be added on the SAME SIDE (cis)
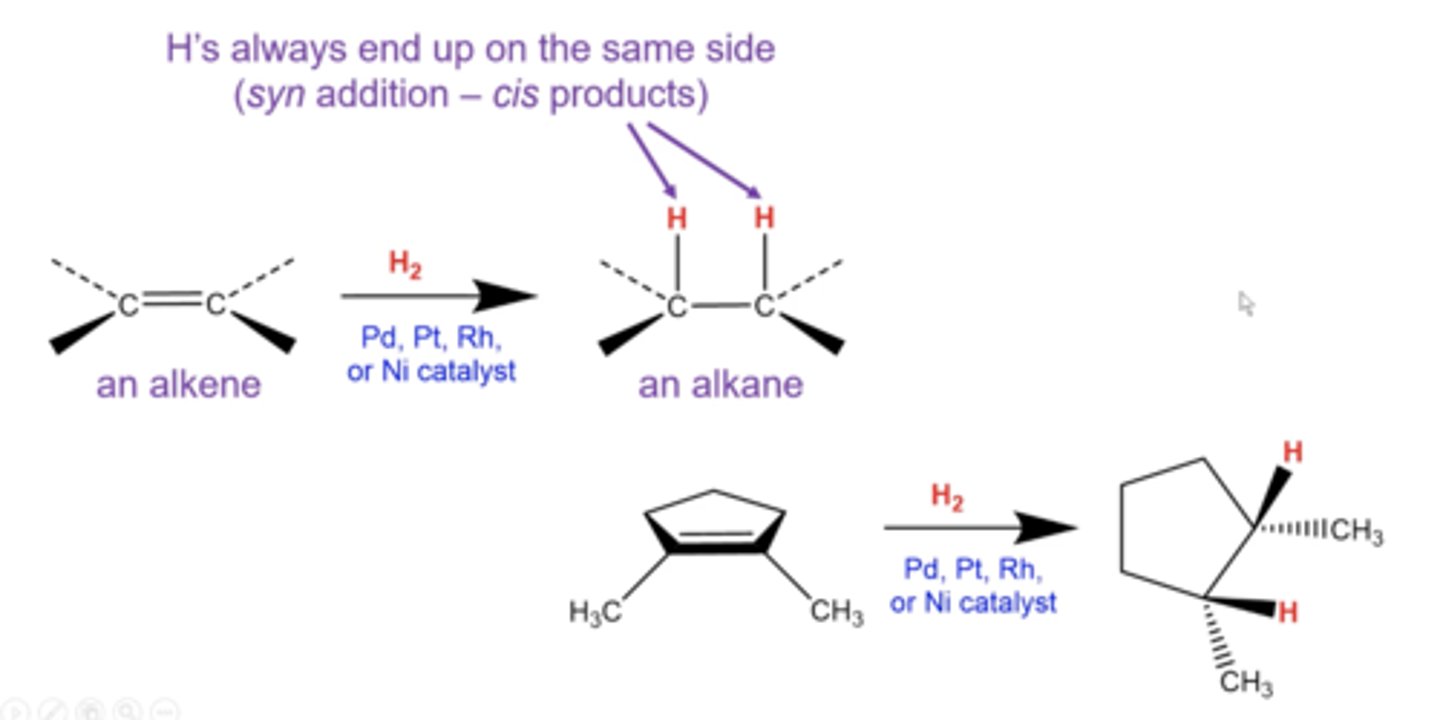
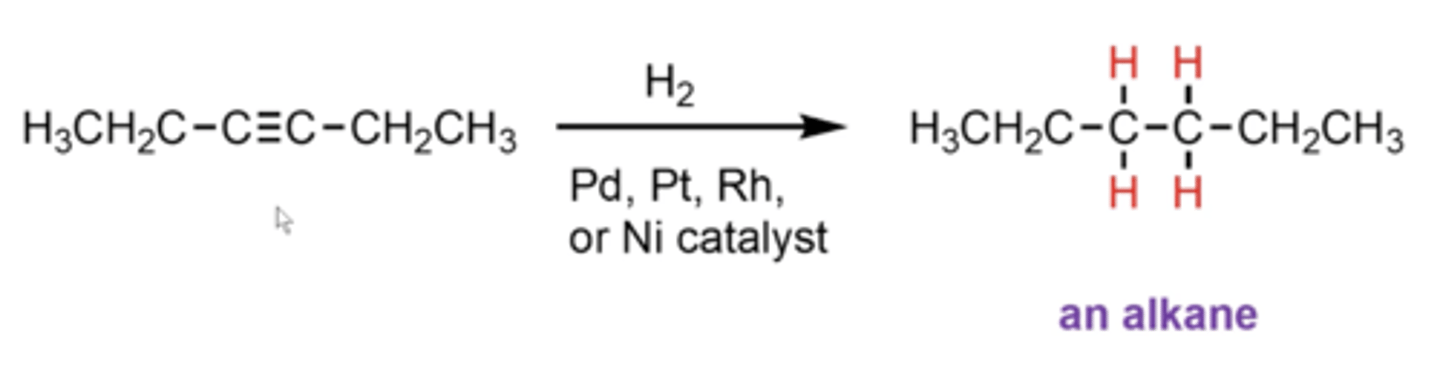
what happens if you take an alkyne and treat it with H2 plus Pd, Pt, Rh, or Ni as a catalyst?
alkynes get reduced all the way down to alkanes
what happens if you take an alkyne and treat it with H2 and Lindlar's catalyst?
you convert the alkyne into a Z-alkene (Z same side)
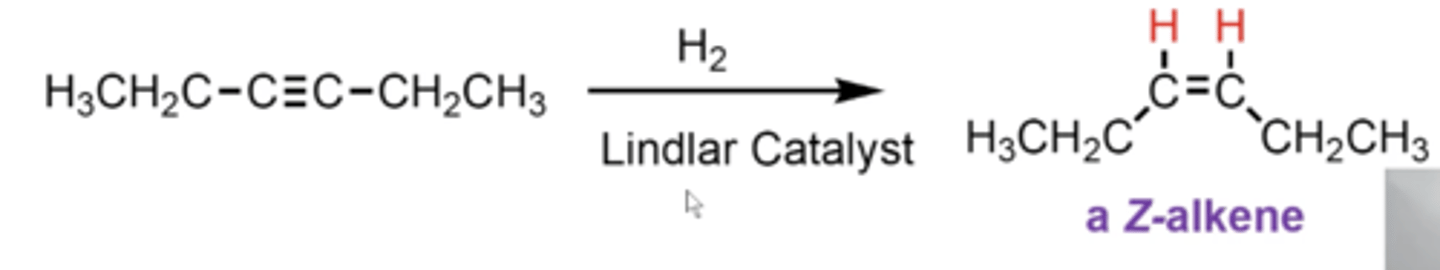
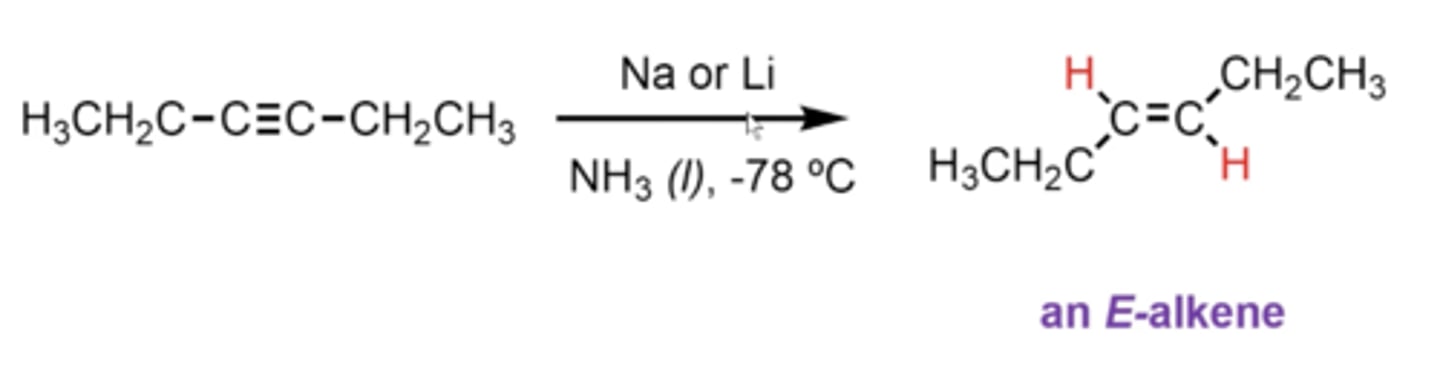
what happens if you take an alkyne and treat it with Na or Li and NH3 (l), -78C
you convert the alkyne into an E-alkene (Eepposite sides)
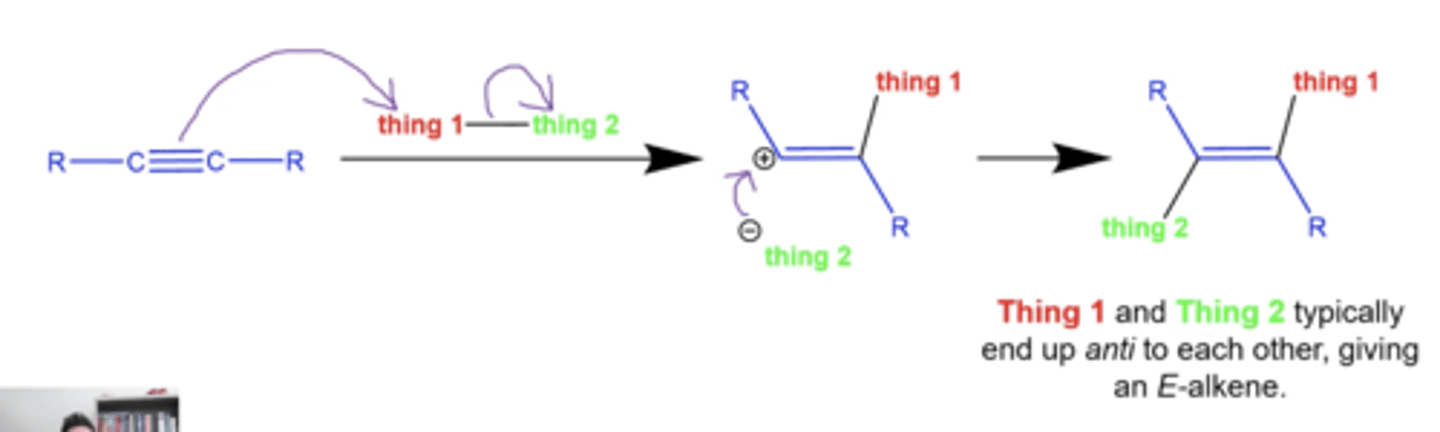
what is the general mechanism of most alkyne reactions?
-usually produces E alkenes (Eepposite sides)
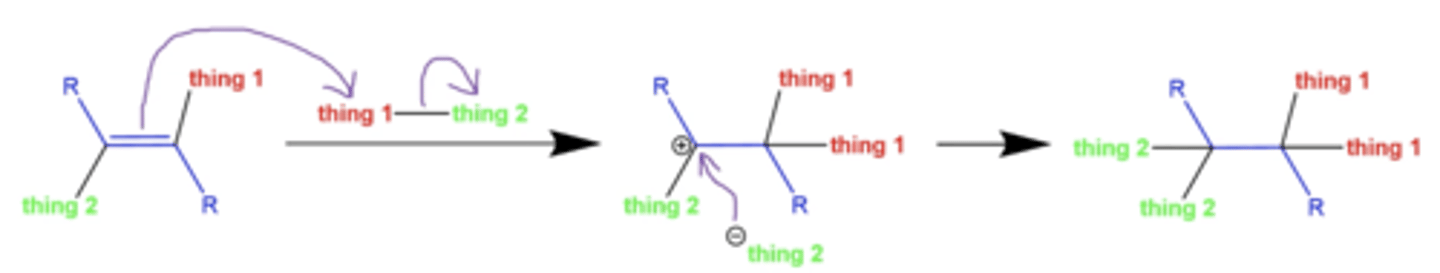
what happens when you add reagents in excess in alkyne reactions?
thing 1 and thing 2 can be added a second time, to produce an alkane
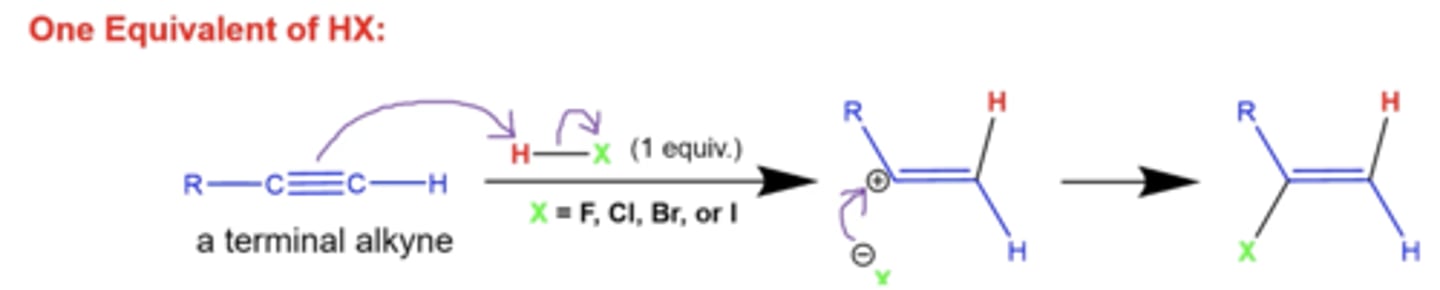
what happens when you treat a terminal alkyne with 1 equivalent of H-X?
X= Cl, Br, F, or I
H gets added to the less substituted side of the alkyne, and X gets added to the more substituted side
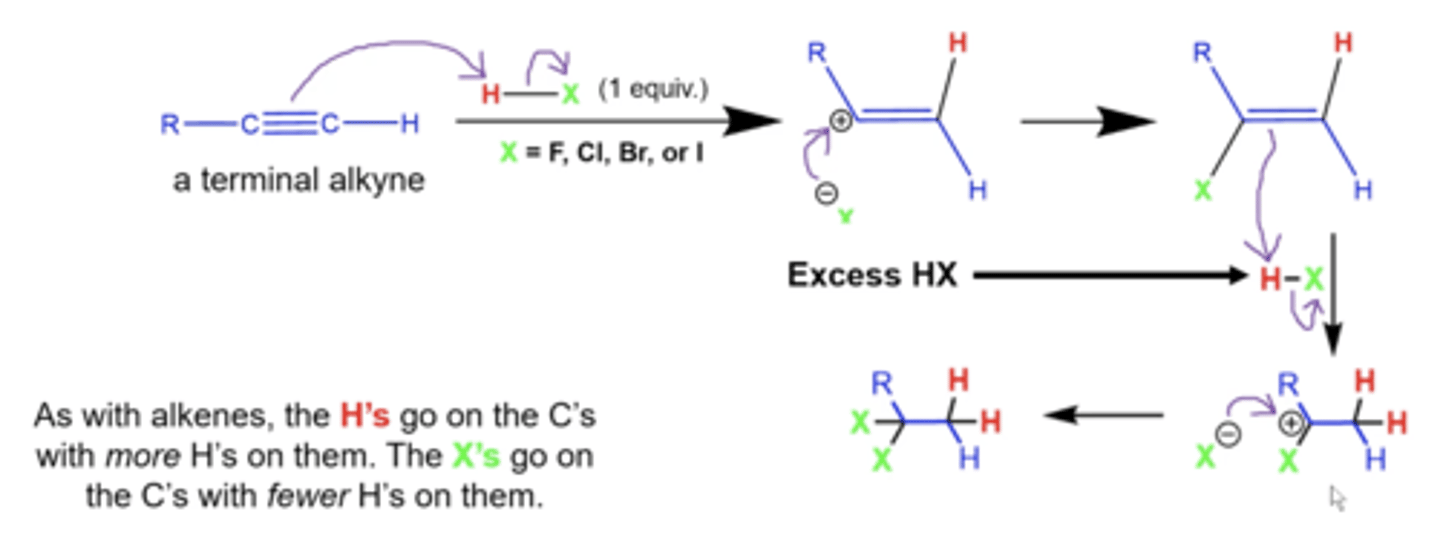
what happens when you treat a terminal alkyne with excess H-X?
X= Cl, Br, F, or I
the alkynes will get completely reduced to alkanes, with the H's going on the less substituted carbon, and the X's go on the more substituted C
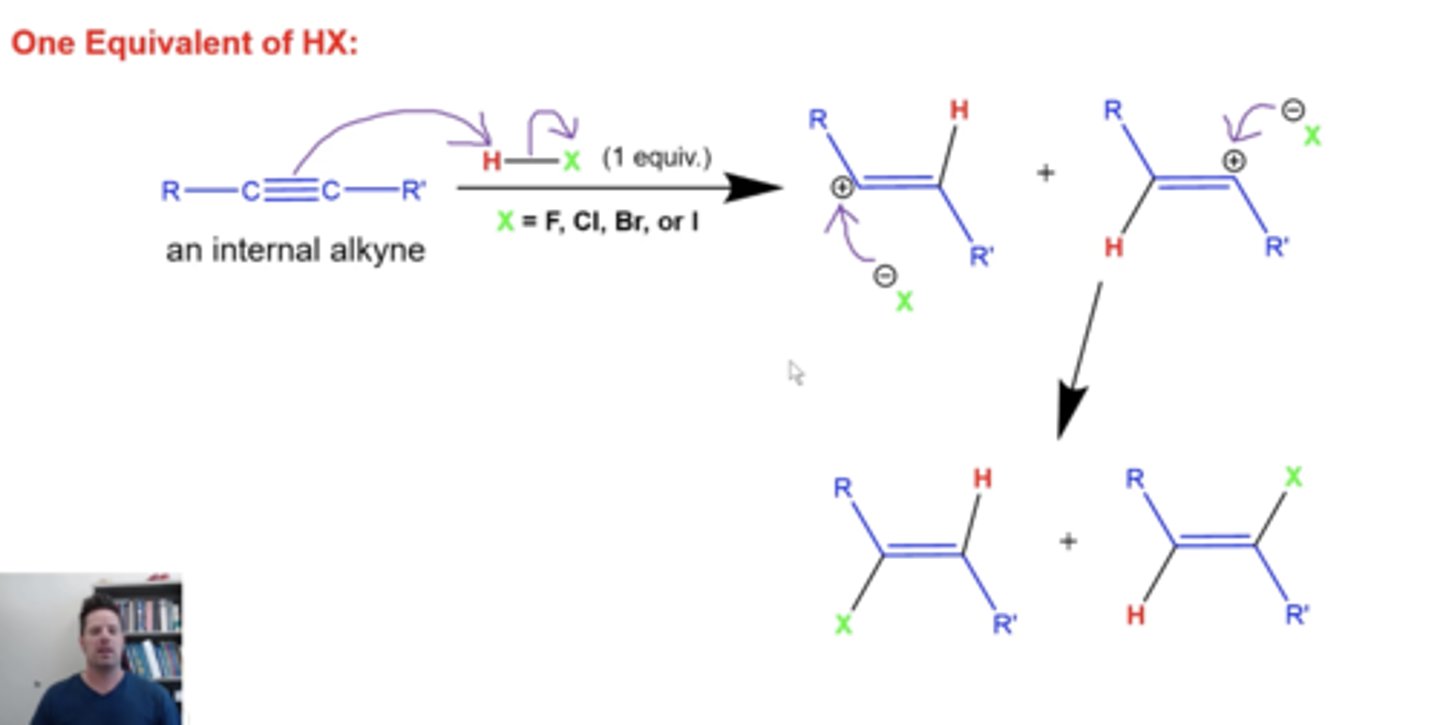
what happens if you add 1 equivalent of H-X to internal alkynes?
because internal alkynes don't form one carbocation with greater stability than the other during addition reactions, adding HX to internal alkynes will give a mixture of products
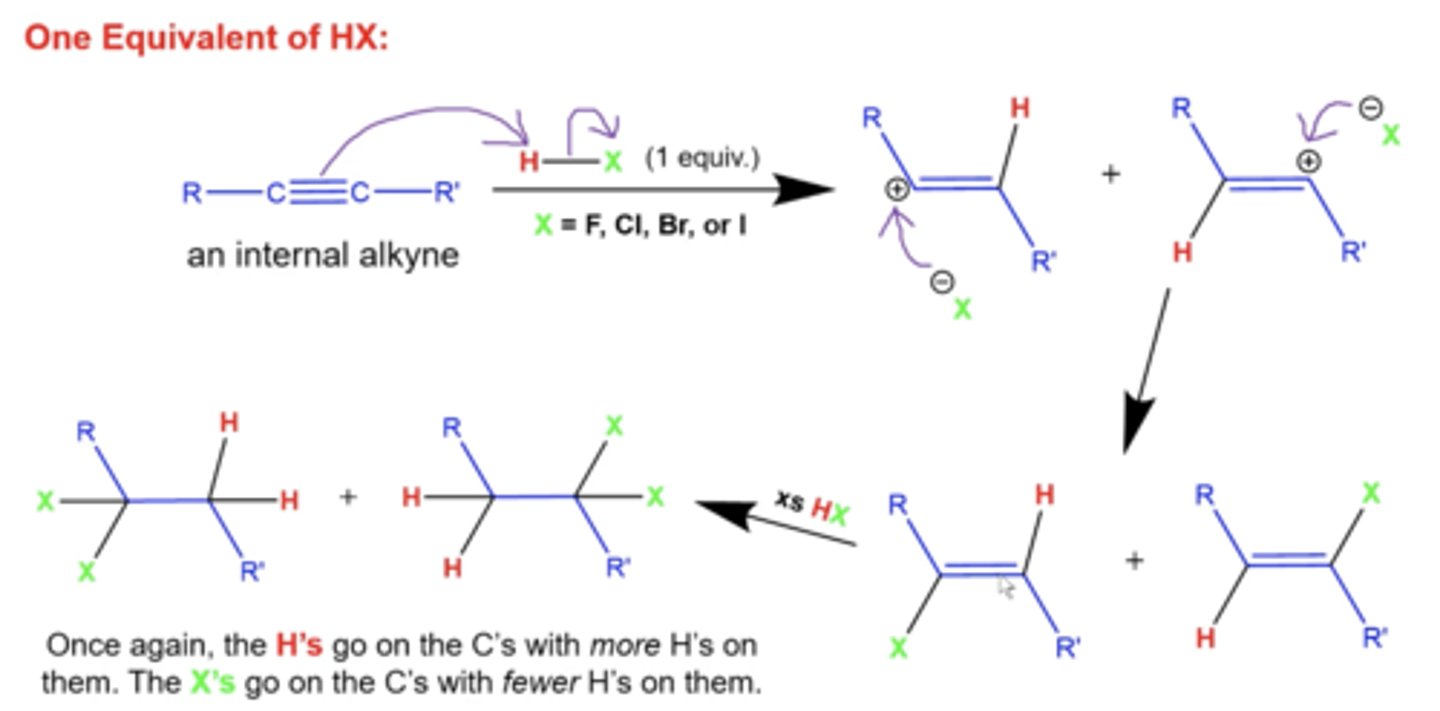
what happens if you add excess H-X to internal alkynes?
they will again form a mixture of 2 products, where the second alkyne
-when going from alkene to alkane, the X's will add to the more substituted carbon and the H's will add to the less substituted carbon
what happens when you add 1 equiv. of X2 to an alkyne?
X= Cl, Br
like alkenes, this will form a 3-membered ring, but with a double bond in the middle, eventually opened up by another X- molecule
-this produces an E alkene
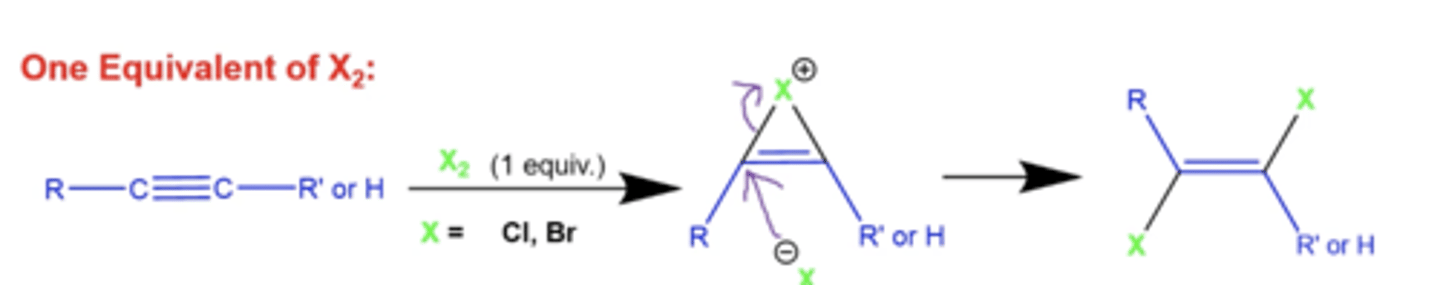
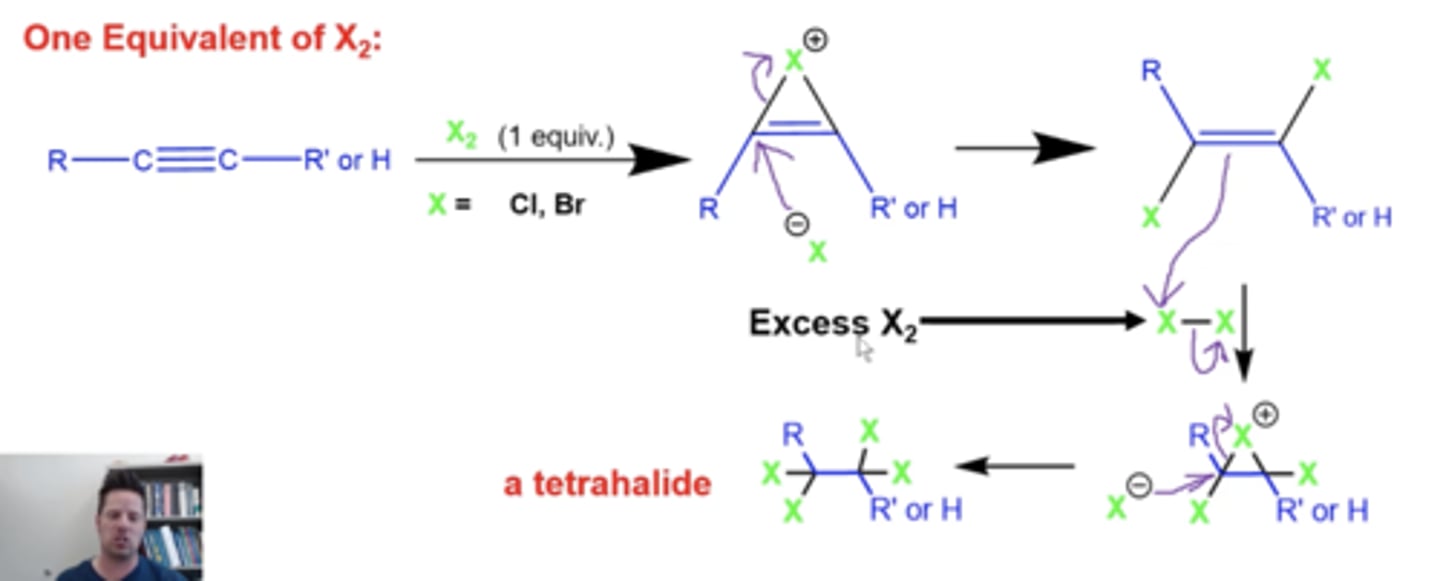
what happens when you add excess X2 to an alkyne?
X= Cl, Br
X2 will add again to the alkene, forming a three-membered ring with all single bonds
-the ring will be opened up by another X molecule, forming a tetrahalide
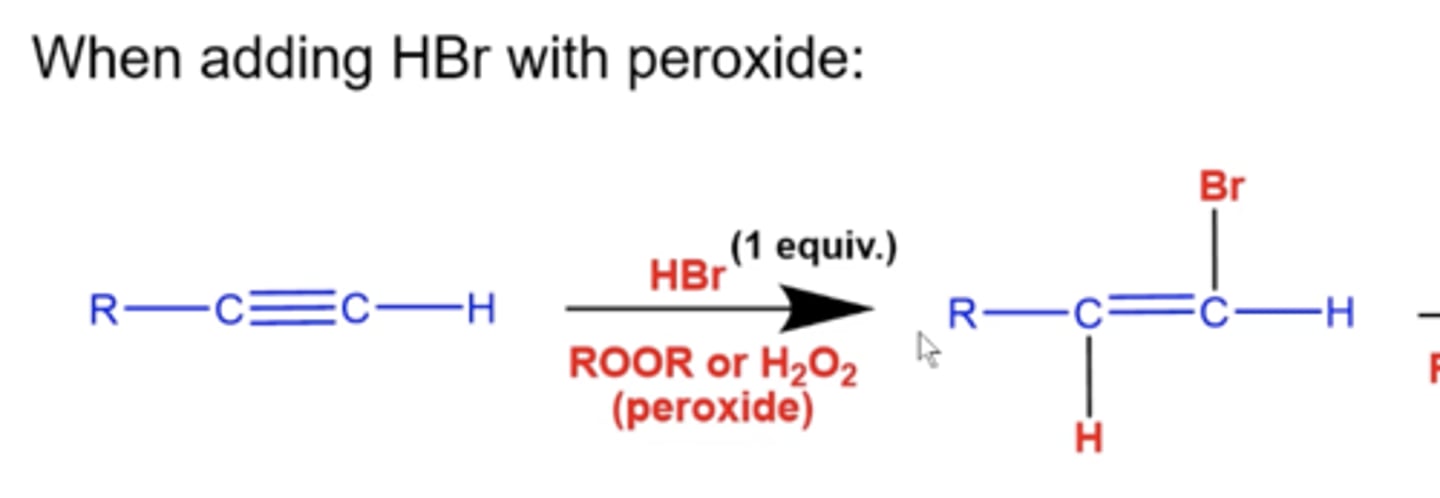
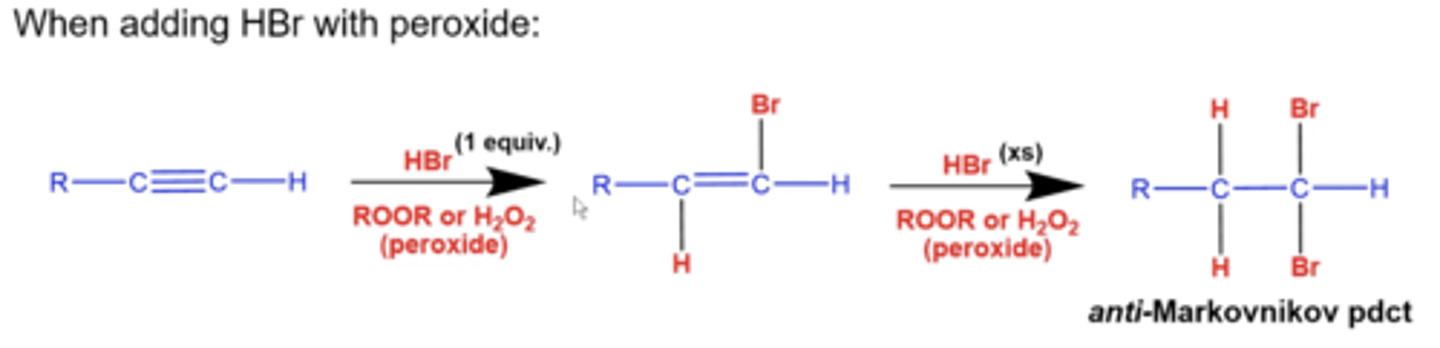
what happens if you add 1 equiv. of HBr and peroxide (ROOR/H2O2) to an alkyne?
you will form the anti-markovnikov product
-Br will add to the less substituted carbon and H will add to the more substituted carbon to form an alkene
what happens if you add excess HBr and peroxide (ROOR/H2O2) to an alkyne?
you will form the anti-markovnikov product
-Br will add to the less substituted carbon and H will add to the more substituted carbon to form an alkane
what happens if you add H2O, H2SO4 (H+ source) and HgSO4 to a terminal alkyne?
you will add an alcohol (OH) and an H:
-H will add to the less substituted carbon and OH will add to the more substituted carbon
(this makes an enol: alkene + alcohol = enol)
-the resulting molecule will undergo tautomerism, where O forms a double bond with the carbon and H swings down (ultimately producing a ketone)
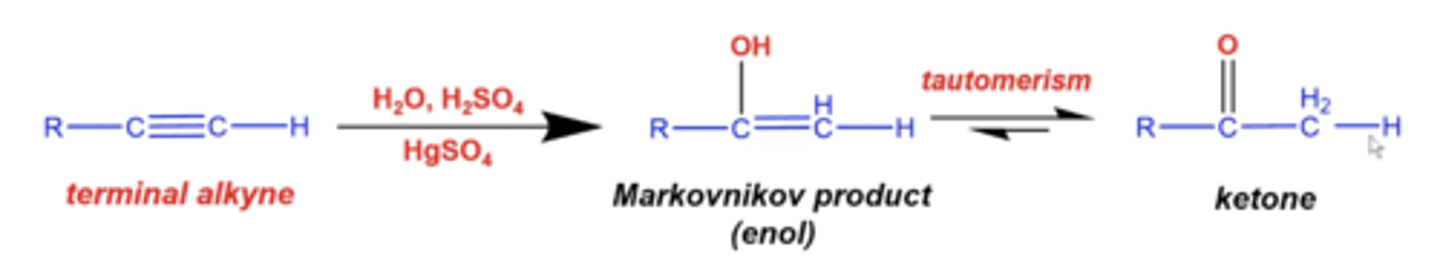
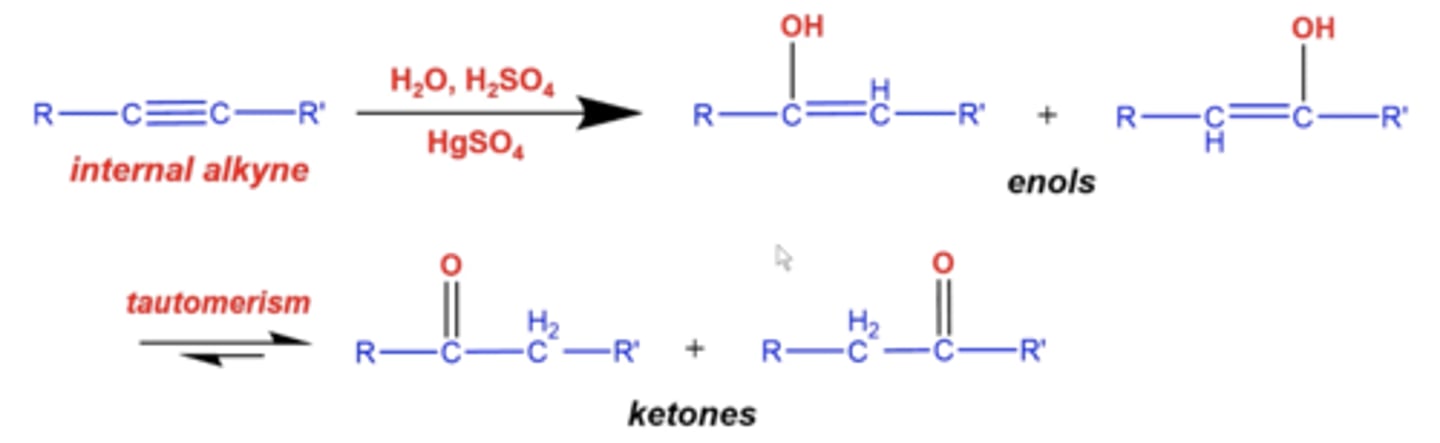
what happens if you add H2O, H2SO4 (H+ source) and HgSO4 to an internal alkyne?
-you will form a mixture of enols where OH randomly adds to either carbon
-both of these enols with tautomerize Into ketones
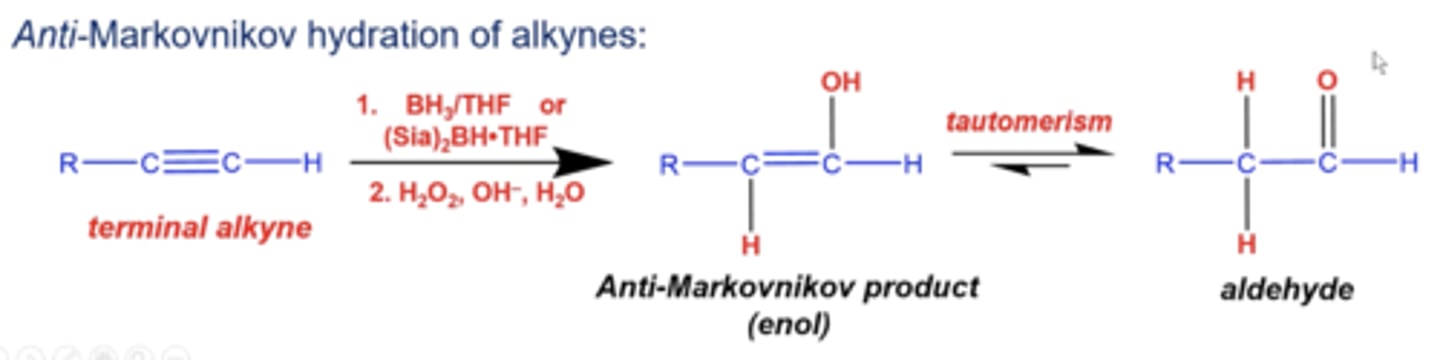
what happens if you add 1. BH3/THF or (Sia)2BH*THF and 2. H2O2, OH-, H2O to a terminal alkyne?
you can form the anti-markovnikov product, where OH adds to the less substituted carbon, and H adds to the more substituted carbon (creating an enol)
-this product undergoes tautomerization and becomes an aldehyde
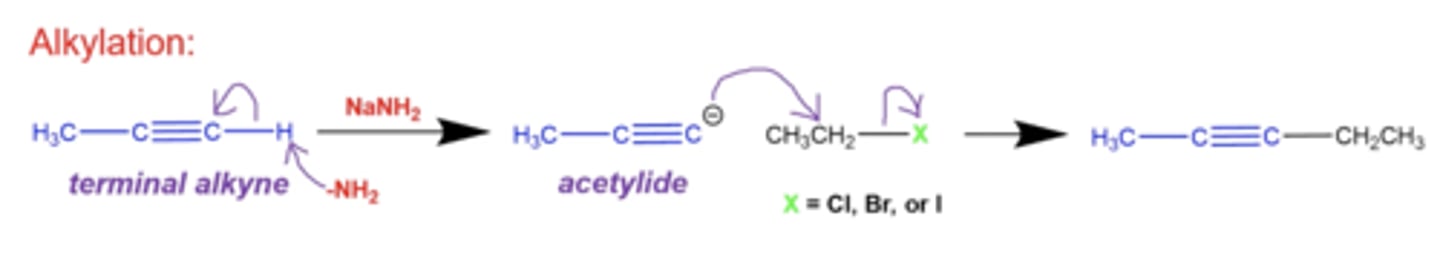
what happens if you add NaNH2 to a terminal alkyne?
-NH2 will kick off a H from the terminal side and form a spot to add an R group
-the R group you can add must be an alkyl halide, where the halide will leave once the R group is inserted into that spot
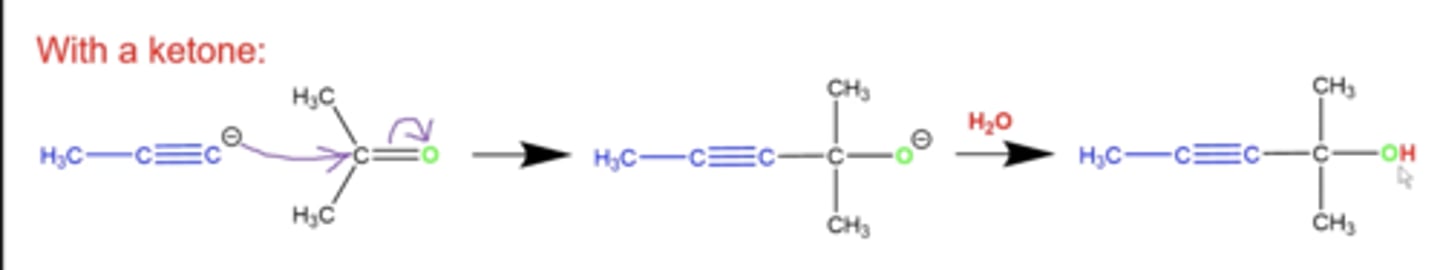
what happens if you react an acetylide (a terminal alkyne with a missing terminal H) with a ketone?
the C- on the terminal alkyne can attack the middle carbon of the ketone, adding the ketone to the alkyne, but with an OH instead of an =O
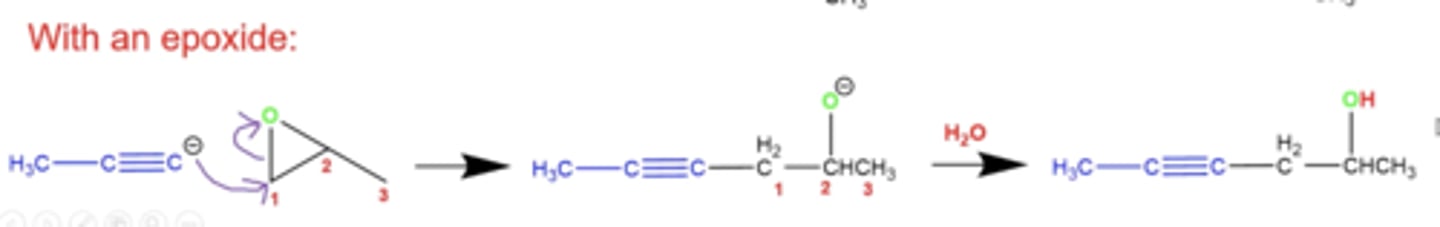
what happens if you react an acetylide (a terminal alkyne with a missing terminal H) with an epoxide?
the C- on the terminal alkyne can attack the most open carbon on the epoxide, opening the epoxide and forming an OH
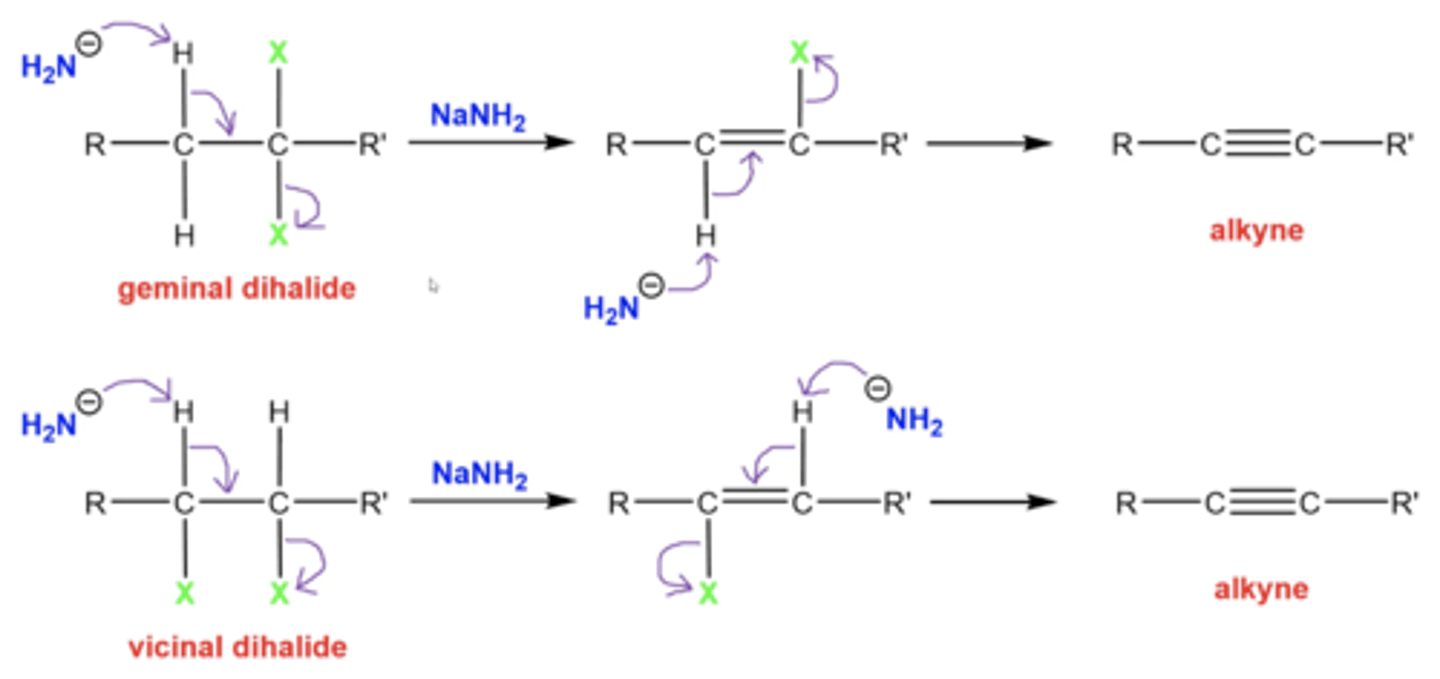
what happens when you add 2 equivalents of NaNH2 to a dihalide? (an alkane with two halogens on either the same carbon or neighboring carbons)
you can form an alkyne
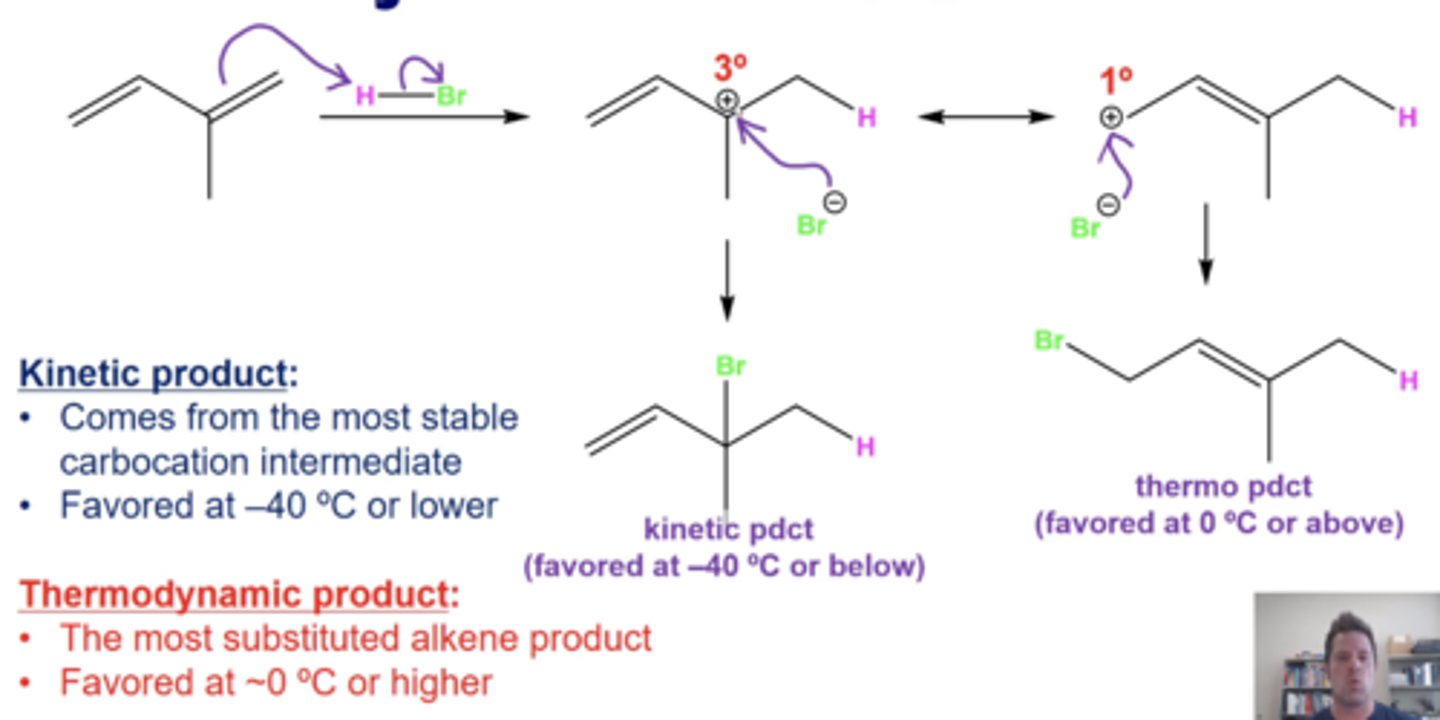
what are characteristics of kinetic products?
-forms fastest
-comes form the most stable carbocation intermediate
-favored at low temperatures (-40C or lower)
what are characteristics of thermodynamic products?
-forms more slowly
-favored at higher temperature (0C or higher)
-is the most substituted (most internalized) alkene product, which is the most stable final product

remember about resonance when forming kinetic and thermodynamic products