Quiz 10: Biomedical Sciences (HL)
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
What is metabolic syndrome in simple terms?
a cluster of metabolic risk factors (obesity, insulin resistance, dyslipidemia, hypertension, glucose dysregulation) that together increase ASCVD risk
How does obesity contribute to metabolic syndrome?
increased fat mass → especially visceral fat → inflammation, insulin resistance, ectopic lipid deposition (liver, pancreas, muscle), and increased BP
What genetic factors can predispose a person to metabolic syndrome?
SNPs in MC4R, leptin deficiency, POMC deficiency, Prader-Willi syndrome, and susceptibility genes that alter hunger, satiety, and fat distribution
How does MC4R dysfunction cause obesity?
loss of MC4R signaling → constant hunger ("feel like they're starving"), increased intake, early-onset severe obesity
Which non-modifiable factors influence metabolic syndrome risk?
age, ethnicity, and family history
How does diet contribute to metabolic syndrome?
excess caloric intake, high-fat/high-sugar diets, fast food, ethanol intake → increased fat mass + insulin resistance
How does physical activity affect metabolic syndrome?
low activity = less energy expenditure → positive fat balance → weight gain
Physical activity is the main modifiable output in energy homeostasis
Which disease states worsen metabolic syndrome?
type 2 diabetes (insulin resistance), hypothalamic obesity (brain injury/tumor), endocrine disorders (Cushing's, hypothyroidism)
How does chronic inflammation from obesity worsen metabolic syndrome?
inflammatory cytokines ↓adiponectin, ↑angiotensinogen → hypertension, endothelial dysfunction, insulin resistance

Why is abdominal ("apple") fat worse than hip ("pear") fat?
visceral fat infiltrates organs → liver fat, fat around heart, pancreatic fat → disrupting organ function & insulin secretion
Why can insulin DECREASE food intake?
in a fed state, insulin signals satiety → activates anorexigenic POMC/α-MSH neurons → decreased hunger
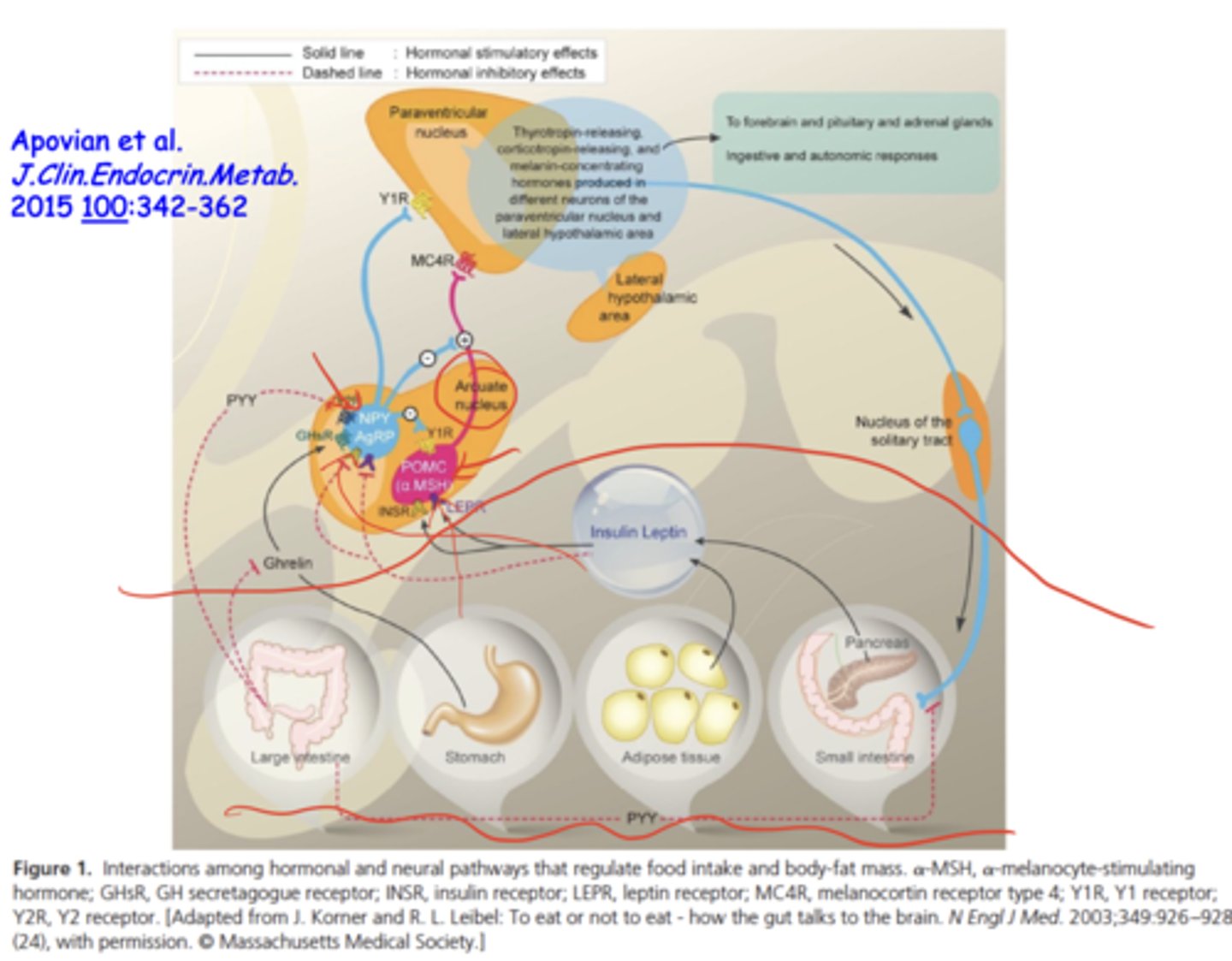
Why can insulin INCREASE food intake?
in insulin-resistant states, exogenous insulin → hypoglycemia → rebound hunger
Also insulin resistance disrupts CNS satiety signaling
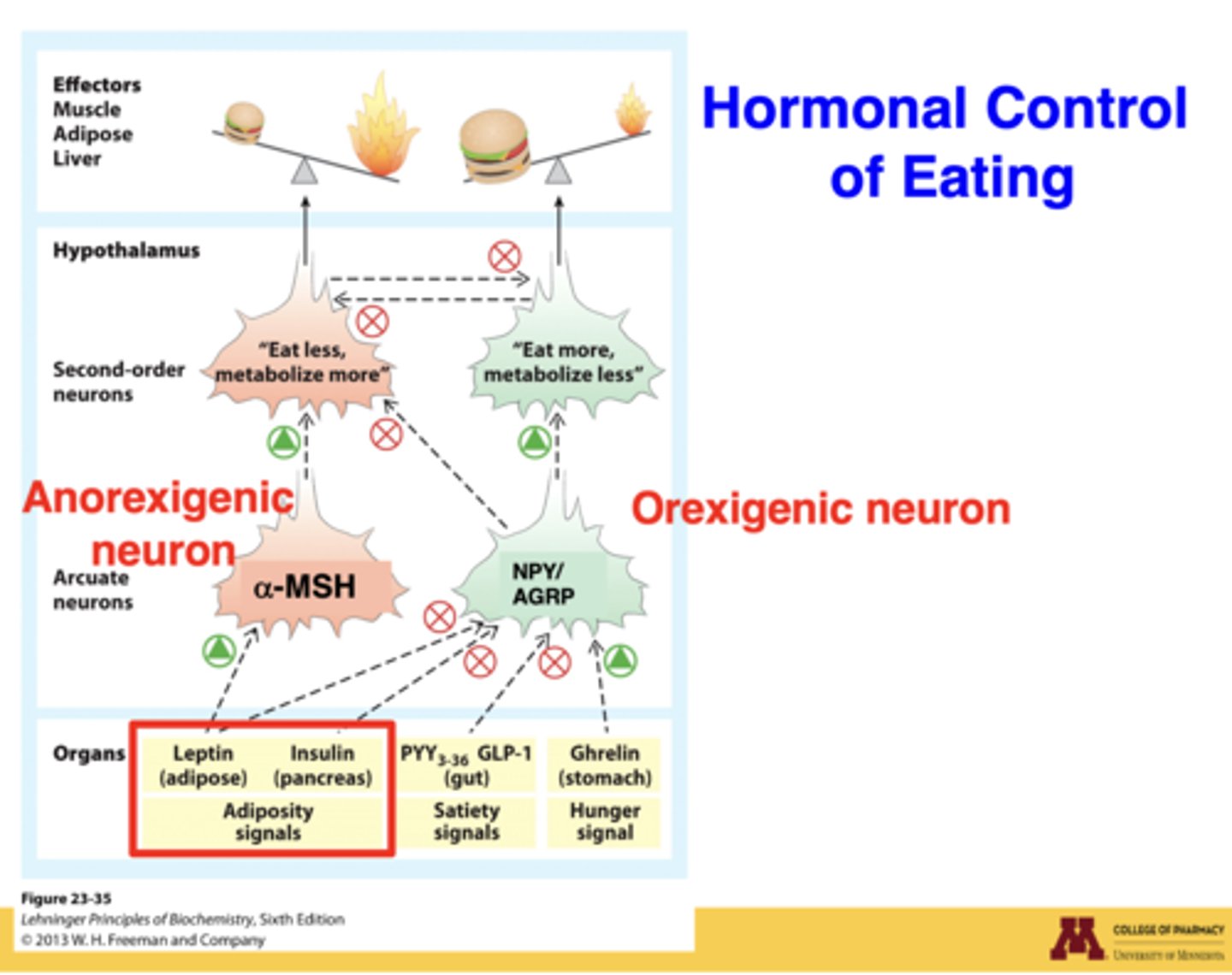
What determines whether insulin increases or decreases appetite?
physiological state (fed vs fasting), insulin resistance, genetics, fat mass, age, and disease states (like diabetes)
How does insulin affect the hypothalamus?
insulin crosses into the brain → interacts with arcuate nucleus (POMC & AGRP neurons) → regulates hunger/satiety
Why do diabetic patients on insulin sometimes gain weight?
exogenous insulin promotes fat storage + compensatory eating during hypoglycemia → increased adiposity
How can caffeine influence metabolic syndrome?
short-term ↑ energy expenditure and ↓ appetite, but tolerance, sleep disruption, and variable effects reduce long-term benefit
How does ephedrine affect weight and metabolic syndrome?
increases thermogenesis and reduces appetite; but long-term effects are inconsistent and adverse CV risks limit use
Why is ephedrine no longer widely used in weight-loss?
variability in effects, safety concerns, and regulatory restrictions following CV events
What's the concern about prescription drugs causing obesity?
many CNS-acting drugs alter feeding pathways (GABAergic, serotonergic, dopaminergic), increasing appetite as a side effect
Why does MSG cause increased hunger ("chinese buffet effect")?
MSG → glutamate → acts as a neurotransmitter → stimulates hunger pathways → increased intake shortly after eating
Why do we question if prescription drugs increase obesity rates?
many modern medications alter CNS feeding signals, and increased longevity means prolonged exposure to these effects
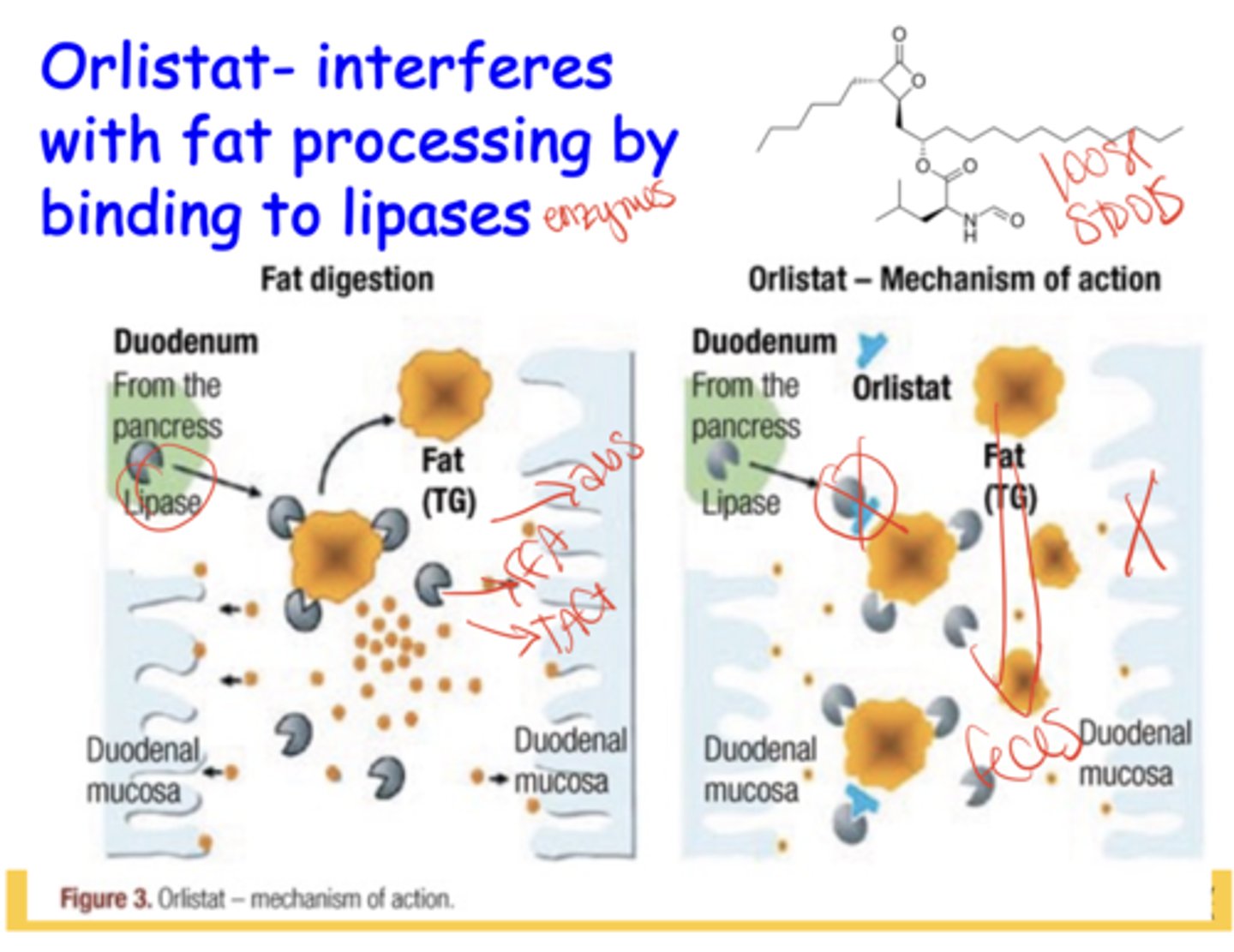
What is the mechanism of action of Orlistat?
inhibits GI lipases → prevents fat breakdown/absorption → fat excreted → causes oily loose stools as major side effect
What receptor does Lorcaserin target?
serotonin 5-HT2C receptor → promotes satiety → decreases appetite.
****withdrawn due to cancer risk

What is the mechanism of Liraglutide/Semaglutide/GLP-1 agonists?
GLP-1 receptor agonists → increase satiety, slow gastric emptying, reduce hunger, regulate POMC pathways in the hypothalamus
How does Phentermine work?
stimulates dopaminergic/adrenergic pathways → appetite suppression
How does Topiramate work?
enhances GABAergic signals → decreases hunger and cravings
Why are phentermine and topiramate used together?
synergy produces significant weight loss
What is the mechanism of Naltrexone?
blocks POMC auto-inhibition (via opioid receptor blockade) → enhances sustained POMC activation
What is the mechanism of Bupropion?
activates POMC neurons → ↑ satiety
Why are naltrexone and bupropion used together?
synergy results in reduced appetite, improved control of cravings
What is the steroid ring system and carbon numbering scheme?
the core steroid structure contains four fused rings (A-D) with carbons numbered 1-27, beginning in ring A and ending on the side chain
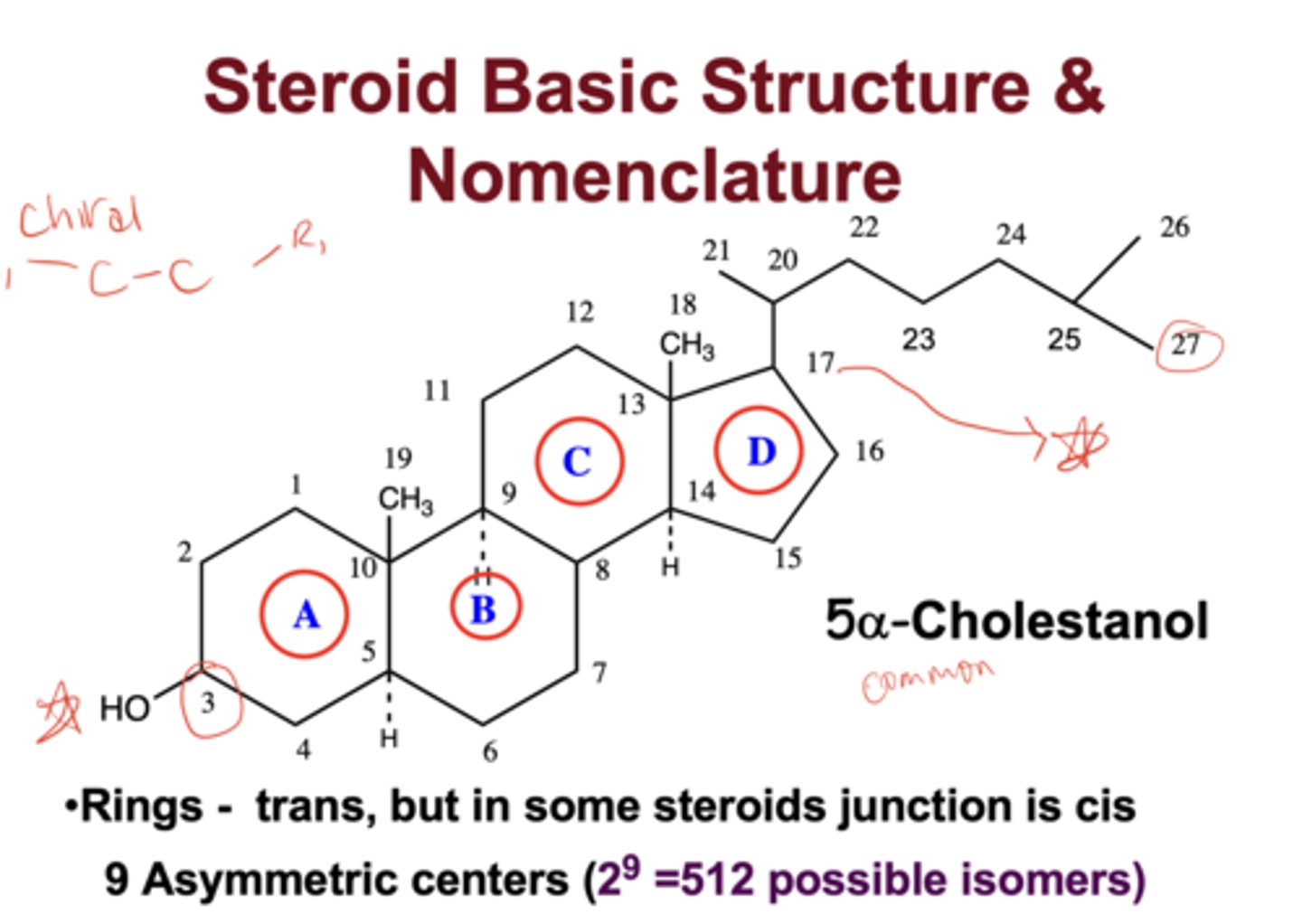
Why is carbon numbering important in steroid biosynthesis?
each enzyme acts at defined carbon positions (e.g., C17, C21), determining the produced hormone class
What is the biosynthetic precursor for all steroid hormones?
Pregnenolone, formed from cholesterol by side-chain cleavage (CYP11A1)
What enzyme converts cholesterol to pregnenolone?
Cytochrome P450 side-chain cleavage enzyme (CYP11A1 / P450scc)
What do hydroxylase enzymes do to a steroid?
insert hydroxyl groups (-OH) at specific carbon positions.
What is the function of 21-hydroxylase?
adds an OH at carbon 21, required for cortisol and aldosterone biosynthesis
Why is 21-hydroxylase clinically important?
deficiency produces congenital adrenal hyperplasia (CAH) with impaired glucocorticoid/mineralocorticoid synthesis
What is the role of 3β-hydroxysteroid dehydrogenase (3β-HSD) in steroid biosynthesis?
3β-HSD converts pregnenolone → progesterone and 17α-hydroxypregnenolone → 17α-hydroxyprogesterone
It is essential for producing glucocorticoids and mineralocorticoids
What is the catalytic role of 17α-hydroxylase (CYP17)?
hydroxylates pregnenolone/progesterone at C17, directing synthesis toward androgens and glucocorticoids
What is the function of aromatase (CYP19)?
converts androgens to estrogens by aromatizing the A-ring
What does 5α-reductase do?
reduces the Δ4-5 double bond, converting testosterone to dihydrotestosterone (DHT)
Why is 5α-reductase important?
it produces DHT, a high-affinity androgen receptor agonist critical for prostate, skin, and genital development
What are the five general principles of steroid metabolism?
1. Oxidation
2. Reduction
3. Hydroxylation
4. Sulfation
5. Glucuronidation
Why does the zona reticularis preferentially produce DHEA?
zona reticularis lacks 3β-HSD, preventing conversion of pregnenolone to progesterone, thereby pushing synthesis toward DHEA and adrenal androgens
What are the main precursors to DHEA and downstream androgens?
pregnenolone → 17-hydroxypregnenolone (CYP17) → DHEA → androstenedione
What is the effect of oxidation on steroid molecules?
introduces oxygen or converts hydroxyls to ketones; often decreases biological activity
What is the effect of reduction on steroid molecules?
alters double bonds or ketone/hydroxyl positions, modifying activity and receptor affinity
What does hydroxylation contribute to steroid metabolism?
increases polarity and prepares steroids for conjugation and renal excretion
What is sulfation in steroid metabolism?
addition of a sulfate group, increasing water solubility
What is glucuronidation in steroid metabolism?
addition of glucuronic acid, producing highly polar metabolites eliminated in urine
What is the rate-limiting step in cortisol metabolism?
reduction of the Δ4-5 double bond by 5α-reductase or 5β-reductase
Why is this Δ4-5 reduction step rate-limiting?
it determines formation of 5α-reduced vs 5β-reduced metabolites and controls overall cortisol clearance
What steroid metabolic pathway is more dominant for cortisol?
5β-reduction, followed by conjugation and urinary excretion
What does the rate-limiting enzyme determine about cortisol clearance?
the proportion of metabolites formed and the efficiency of cortisol inactivation
What are the major tissues responsible for steroid metabolism and excretion?
liver (biotransformation) and kidney (elimination of conjugates).
What structural modification distinguishes estrogens from other steroids?
an aromatic A-ring, produced by aromatase
What defines cortisol synthesis in the zona fasciculata?
presence of 17α-hydroxylase (CYP17) and 11β-hydroxylase (CYP11B1) enabling pregnenolone → 17-OHP → 11-deoxycortisol → cortisol
Which steroid is uniquely synthesized in the zona glomerulosa and why?
aldosterone, because the zona glomerulosa uniquely expresses aldosterone synthase (CYP11B2) and lacks CYP17
What is the pathway from progesterone to aldosterone?
progesterone → CYP21 → deoxycorticosterone → corticosterone → CYP11B2 → aldosterone
What property of steroids explains why they are not stored in vesicles?
they are lipophilic and diffuse freely; therefore synthesized on demand
What determines the type of steroid hormone produced in each adrenal zone?
the enzyme expression profile of the tissue (e.g., CYP17 absent in zona glomerulosa → no cortisol/androgen synthesis)
What is the HPA axis and why is it important for steroid biosynthesis?
regulates steroid biosynthesis by controlling CRH release from the hypothalamus, ACTH secretion from the pituitary, and adrenal cortisol production
What does ACTH (corticotropin) have to do with steroid biosynthesis?
ACTH stimulates adrenal steroidogenesis by increasing cholesterol transport into mitochondria and upregulating steroidogenic enzymes
What are the general physiological functions of steroid hormones?
metabolic regulation, electrolyte balance, reproductive function, inflammatory and stress responses, bone metabolism, cardiovascular regulation, and neurobehavioral effects
What are three adrenal diseases discussed?
Addison' Disease, Cushing's Disease/Syndrome, and Congenital Adrenal Hyperplasia
Addison's Disease
primary adrenal insufficiency due to low cortisol/aldosterone and high ACTH
Cushing's Disease/Syndrome
excessive ACTH or cortisol leading to high adrenal activity
Congenital Adrenal Hyperplasia
enzyme deficiency (commonly 21-hydroxylase) leading to impaired cortisol synthesis and excess androgens
What are five common problems that can be treated with steroid drugs?
inflammatory diseases, allergic disorders, autoimmune disorders, dermatologic conditions, pulmonary diseases (e.g., asthma/COPD)
What are five common side effects associated with steroid drugs?
immunosuppression, hyperglycemia, osteoporosis, Cushingoid changes (e.g., moon face), and adrenal suppression
What structural change in prednisone/prednisolone increases anti-inflammatory potency and half-life?
C1-C2 double bond in the A-ring decreases metabolic reduction → increases stability and half-life (2-4 hrs to 60min)
How does the C1-C2 double bond alter glucocorticoid receptor selectivity?
It shifts activity toward greater glucocorticoid potency relative to mineralocorticoid potency
What structural modification in methylprednisolone increases its half-life compared to prednisolone?
It has a 6-α-methyl group, which increases glucocorticoid receptor selectivity and slows hepatic metabolism, extending duration of action (16-26 hrs)
What structural differences make dexamethasone long-acting?
2 key modifications:
- 9-α-fluoro substitution, which increases receptor affinity and potency
- 16-α-methyl group, which blocks metabolic inactivation
result → long half life (190mins)
What structural characteristics determine hydrocortisone's (cortisol's) half-life?
It has no synthetic structural modifications. Since it retains the native cortisol structure, it is rapidly metabolized by hepatic reduction and conjugation → short half-life
What structural differences in cortisone affect its half-life compared with hydrocortisone?
It has a keto group at C11 instead of a hydroxyl. It must be enzymatically converted (11β-HSD1) to active cortisol; however, it still lacks stabilizing modifications, so its half-life remains short, similar to hydrocortisone
Are steroid-based drugs used for inhalation and dermatologic treatments?
Yes, specific corticosteroids are formulated for inhaled use and topical dermatologic use to achieve local anti-inflammatory effects
Plasma Half-Life
the time required for plasma concentration of a drug to decrease to half its initial value
Biological Half-Life
the time required for half of a drug to be removed from the body via metabolism, conjugation, and excretion