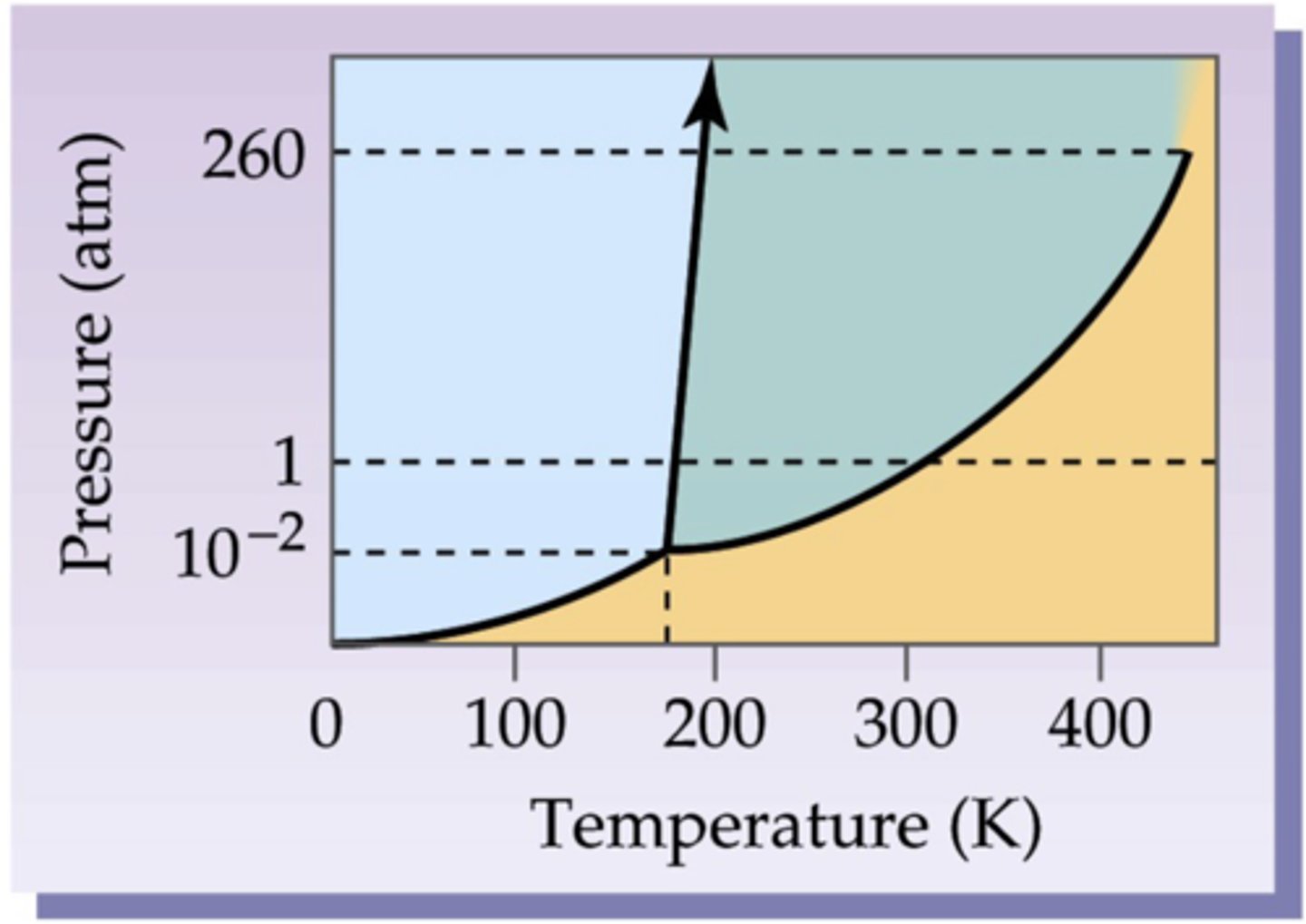
sublimation, vaporization , condensation, deposition, melting, freezing
0.0(0)
Card Sorting
1/13
There's no tags or description
Looks like no tags are added yet.
Last updated 6:32 PM on 9/10/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
1
New cards
From solid to gas
sublimation (absorb energy)
2
New cards
from gas to sold
deposition (release energy)
3
New cards
from solid to liquid
melting
4
New cards
from liquid to solid
freezing
5
New cards
from liquid to gas
vaporization
6
New cards
from gas to liquid
condensation
7
New cards
sublimation exo or endo
endothermic
8
New cards
vaporization
endothermic
9
New cards
melting
endothermic
10
New cards
deposition
exothermic
11
New cards
condensation
exothermic
12
New cards
freezing
exothermic
13
New cards
what are some chemicals that do not equal 0 for thermodynamics?
C,H,O,S
14
New cards
where is normal melting and boiling point
at the 1 mark for both