CBNS lectures 13-15 filaments
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
110 Terms
what are the 3 classes of cytoskeletal filaments?
Microtubules (Largest) - Hollow tubes, help with cell shape, transport, and division.
Microfilaments (Smallest) - Made of actin, help with cell movement and shape.
Intermediate Filaments (medium-sized) - Give strength and durability to cells.
where do microtubules emanate from?
the centrosome, usually near nucleus
Determine the position of membrane-enclosed organelles and direct intracellular transport
where do Intermediate filaments extend through?
throughout the cytoplasm and form a lamina beneath the nuclear membrane.
Provide mechanical strength and resistance to shear stress
where does the microfilaments/ actin filaments tend to be
organized into networks associated with the cell periphery (cortex).
Determine the shape of the cell’s surface and are necessary for whole-cell locomotion
these cytoskeletal filaments cannot what?
function on their own…
they depend on accessory proteins that link the filaments to the other cell components, as well as to each other.
These accessory proteins are essential for the assembly of the cytoskeletal filaments, in particular their position, and they include the motor proteins that move organelles along the filament (or move the filaments themselves)
FUNCTIONS of MICROTUBULES
they organize cellular organelles within cytoplasm.\
provide tracks on which cell constituents move.
provide the force for Cilla and flagella beating
form the mitotic spindle
Functions of actin filaments / microfilaments
organize in a variety of linear bundles, 2D sheets or 3d gels, and are mainly found underneath the plasma membrane – the cell cortex
these push and pull the plasma membrane resulting in shape changes,
assemblies provide muscle contaction
Functions of intermediate filaments
components of the nuclear lamina, that may help to organize chromatin within the nucleus.
Control nuclear disassembly /reassembly during mitosis.
provide mechanical integrity to tissues, that are subject to mechanical stress
Example of (rapid) cytoskeletal reorganization: cell division
Actin filaments help the cell move and later form the contractile ring to split the cell.
✅ Microtubules guide the process by forming the mitotic spindle, which separates chromosomes.
✅ The cytoskeleton completely reorganizes itself before, during, and after division to ensure a smooth transition into two separate daughter cells.
An in vitro model for polymerization of assembly subunits to form a cytoskeletal filament (polymer)
Filaments only grow or shrink at the ends—not in the middle.
✅ The first step (nucleation) is slow and requires extra energy. form small clusters
✅ Once the filament starts growing, subunits add and remove quickly for rapid reorganization.
✅ Weak bonds between subunits make the filament stable but still flexible. ie. hydrophobic interactions and weak, non-covalent ionic bonds
critical concentration (Cc)
is the minimum concentration of free subunits needed for filaments to grow.
If the concentration of free subunits is higher than Cc,
the filament will grow (net assembly).
If the concentration of free subunits is lower than Cc,
the filament will shrink (net disassembly).
When the concentration of free subunits equals Cc,
the filament is at steady state (no net growth or shrinkage).
How Do Subunits Add?
Adding Subunits (Polymerization)
The rate at which subunits attach to the filament is proportional to the concentration of free subunits.
they will bump into the filament more often and have a higher chance of attaching.
More free subunits = faster growth.
Formula: KON * C (where KON is the "on-rate" constant and C is the free subunit concentration).
how do subunits leave?
Removing Subunits (Depolymerization)
The rate at which subunits detach from the filament is constant and does not depend on concentration.
Subunits leave at a steady rate no matter how many free subunits are available.
Formula: KOFF (where KOFF is the "off-rate" constant).
plus and minus ends kinetics of microtubules or microfilaments
even tho the bonds are the same
(+) ends grow faster and subunits fit into the filament more easily due to their shape and conformation.
(-) ends grow slowly because of a more difficult conformational change assembly
Above Cc → both ends grow, but plus end grows faster.
✅ Below Cc → both ends shrink, but minus end shrinks faster.
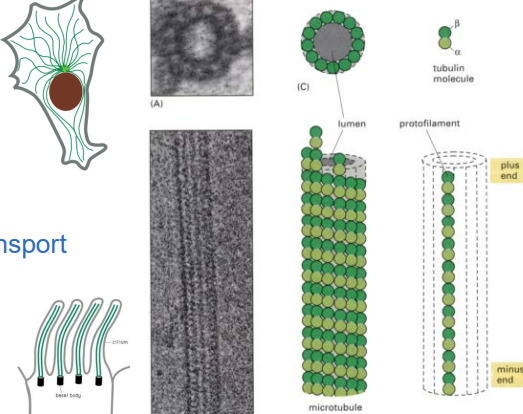
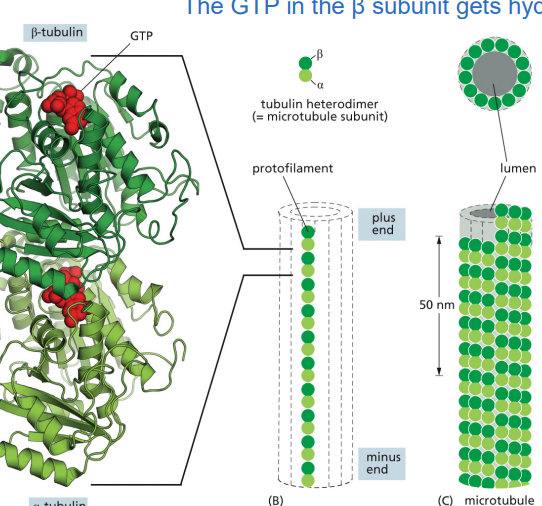
The microtubule assembly subunit is a
α-tubulin/β-tubulin heterodimer
✅ Only the β-tubulin subunit hydrolyzes GTP into GDP, which regulates stability.
✅ GTP-bound tubulin is stable and promotes growth, while GDP-bound tubulin is unstable and promotes disassembly.
a long chain of heterodimer tubulins is called a protofillament
The microfilament assembly subunit is a
actin monomers
✅ Actin binds ATP when free and hydrolyzes it to ADP after joining a filament.
✅ ATP-actin is more stable (promotes growth), while ADP-actin is less stable (promotes disassembly).ATP is slowly hydrolyzed into ADP.
Freshly added actin subunits are ATP-actin (more stable).
Older actin subunits inside the filament are ADP-actin (less stable, more likely to disassemble).
✅ This ATP-to-ADP switch drives actin filament dynamics, allowing cells to change shape and move.
What is the role of nucleotide hydrolysis in microtubule assembly
GTP binding is needed for microtubule assembly, but hydrolysis controls stability.
✅ Hydrolysis weakens microtubules, making GDP-tubulin more likely to detach.
✅ If GTP addition is faster than hydrolysis → microtubule grows.
✅ If hydrolysis catches up → microtubule shrinks (catastrophe).
✅ The minus (-) end has more trouble keeping up with assembly compared to the plus (+) end.
Microtubule treadmilling
✅ Treadmilling happens when the plus end grows while the minus end shrinks.
✅ GTP-tubulin is more stable and assembles more easily (low Cc), while GDP-tubulin is unstable and disassembles quickly (high Cc).
✅ If the free tubulin concentration is between the Cc values of both ends, treadmilling occurs.
✅ This allows microtubules to remain dynamic without excessive growth or shrinkage.
boiling point analogy vs Critical concentration
GTP-tubulin assembles, GDP-tubulin disassembles.
✅ Cc(GDP) > Cc(GTP), meaning GDP-tubulin disassembles more easily.
✅ Treadmilling = Plus end grows while minus end shrinks.
✅ Dynamic instability = Some plus ends grow while others rapidly shrink.
✅ This allows microtubules to be highly dynamic and adaptable.
GTP tubulin has a “lower boiling point” meaning less filaments will still bind easier and better
The Importance of the GTP Cap in Mts
Microtubules grow when GTP-tubulin is added faster than GTP is hydrolyzed.
This creates a GTP cap at the plus end, which keeps the microtubule stable and growing.
If the GTP cap is lost (because GTP gets hydrolyzed to GDP before new GTP-tubulin arrives), the microtubule undergoes catastrophic shrinkage (disassembly).
GTP-bound tubulin (straight protofilament, stable)
Tubulin dimers in the GTP state have a straight conformation.
This allows them to pack tightly and form stable microtubules.
When GTP-tubulin is added faster than GTP is hydrolyzed, the microtubule maintains a GTP cap, keeping it stable and growing.
GDP-bound tubulin (curved protofilament, unstable)
When GTP is hydrolyzed to GDP, the tubulin undergoes a conformational change and becomes curved.
Curved protofilaments cannot hold together well, causing the microtubule to fall apart (depolymerization).
If a microtubule loses its GTP cap, it rapidly shrinks in an event called catastrophe.
Tubulin and actin have been highly conserved during eukaryotic evolution
Both are present in all eukaryotic cells Both have multiple isoforms / are encoded by multiple genes:
In mammals, there is at least 6 α- and βtubulins 6 different actins in humans Used in distinct locations and can perform subtly different functions
Variations between isoforms are expected to affect the association of accessory proteins rather than polymerization
Intermediate filaments specialized !!
specialized for structural support, not movement.
✅ They are found in cells that need extra strength, like skin, muscle, and nerve cells.
✅ They don’t grow or shrink rapidly like microtubules or actin filaments.
✅ They provide resilience to cells under mechanical stress.
Have rope-like mechanical properties useful to soft-bodied animals, such as nematode
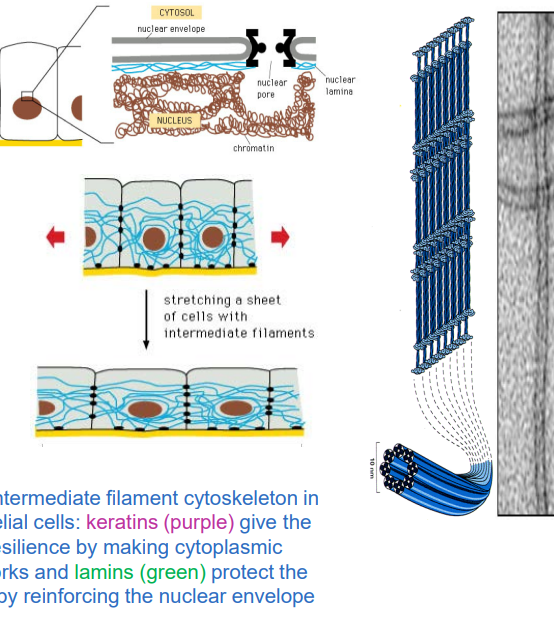
IF’s :Nuclear lamins
Nuclear lamins form a meshwork inside the nucleus, called the nuclear lamina.
✅ They provide structural support, anchor DNA, and maintain nuclear pore stability.
✅ They break down and reform during cell division.
✅ Lamin genes evolved to give rise to both nuclear and cytoplasmic intermediate filaments.
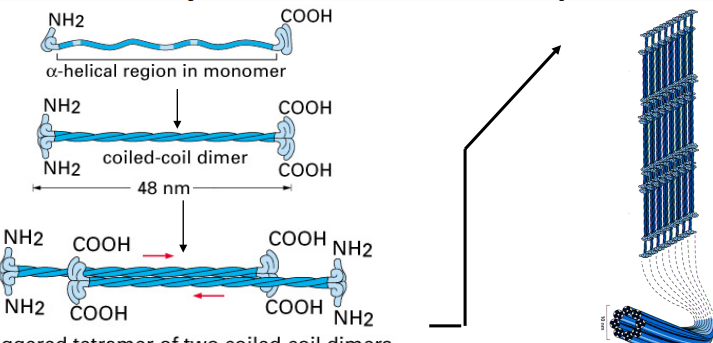
Intermediate Filament (IF) Assembly Unit
Each IF protein has a central helical domain that allows it to coil around another IF protein, forming a dimer.
Two dimers then pair up in an opposite (antiparallel) orientation to form a tetramer.
this is the form that floats around in the cytoplasm before being assembled into a filament
The lateral interactions between IF subunits give intermediate filaments fibrous properties similar to a rope
intermediate filament terminals
All IF proteins have a similar coiled-coil structure with variable amino and carboxyl terminal regions
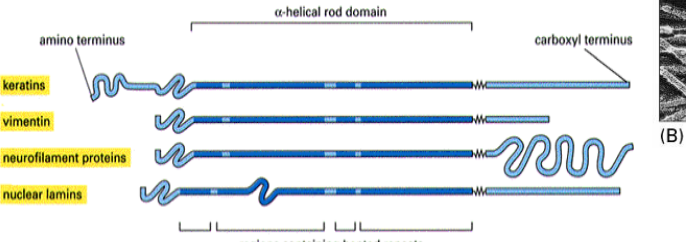
wat is the most diverse type of IF?
Keratins
20 found in different types of human cells
10 specific to hair and nails
where are keratins usually present?
epithelial cells where they are indirectly connected to its neighboring cells
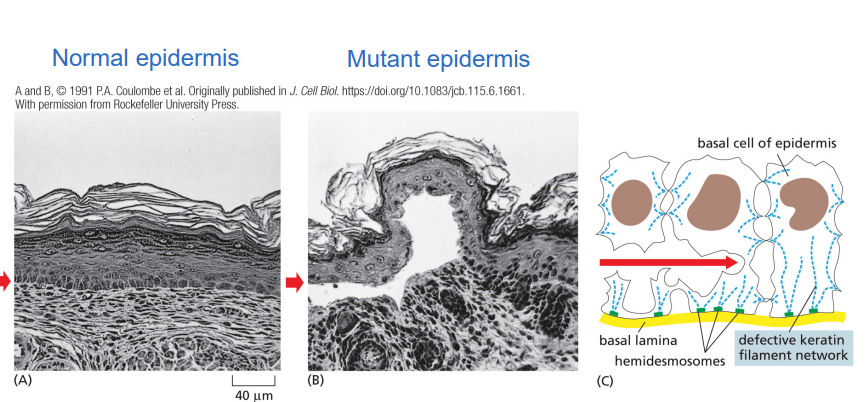
what happens if there is a mutation in cytokeratin IF protein?
the epidermis is prone to tear and blister in response to mechanical stress
what type if Intermediate Filaments (IFs) are found inside axons of vertebrate neurons?
Neurofilaments
Neurofilaments & Axonal Growth mechanism
During neuronal development and repair, neurofilaments are continuously added along the axon in a dynamic process.
How Do Neurofilaments Affect Signal Transmission?
More neurofilaments = a thicker axon.
A thicker axon = faster electrical signal transmission.
This means the level of neurofilament expression directly affects how quickly nerve impulses travel.
In some neurons, neurofilaments are the major determinant of axon diameter, helping optimize communication speed in the nervous system.
Amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease)
linked to abnormal neurofilament assembly in motor neurons.
Neurofilaments accumulate abnormally in neuron cell bodies and axons, which may block transport of essential molecules and interfere with normal neuron function.
This results in axon degeneration, muscle weakness, and eventual paralysis, which is usually fatal.
how do nuclear Lamins disassemble like during mitosis ?
Via Cdc2 kinase, it adds a phosphate group.
this causes shape changes and then a phosphatase removes it once it is ready to reassemble
Cytoskeletal Filaments and Toxins
plants or fungi often produce toxins as a defense mechanism, these prevent filaments from forming, bind to a filament of a free subunit to form a polymer
TAXOL
extracted from the bark of a yew tree, binds to and stabilizes microtubules causing a net increase in polymerization
Taxol is widely used for cancer treatment. Preferably kills dividing cells.
What Does the Cell Control?
Cells regulate everything about their cytoskeleton, including:
Length – Making filaments longer or shorter as needed.
Stability – Keeping filaments stable or breaking them down.
Number – Increasing or decreasing the amount of filaments.
Geometry – Controlling their shape and arrangement.
Connections – Attaching them to other filaments or structures inside the cell.
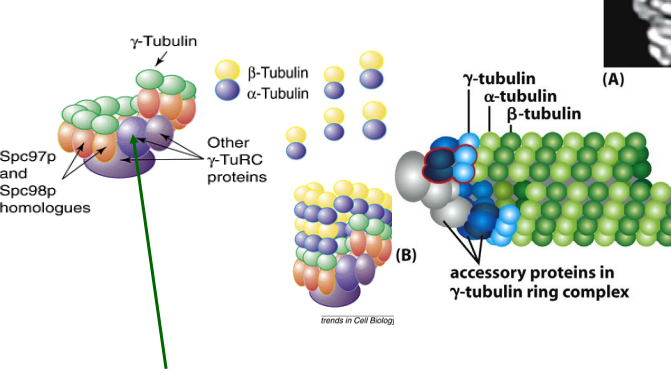
Microtubules are mainly made up of a-/b- tubulin but starts with what?
γ-tubulin ring complex
γ-TuRC acts like a platform that helps new microtubules start growing.
It removes the barrier to nucleation, making microtubule formation much easier.
Microtubule Organizing Center (MTOC)
the centrosome that acts like a microtubule factory,
anchored at their minus ends in the centrosome, while their plus ends extend outward, growing toward different parts of the cell.
What is Colchicine, and How Does It Affect Microtubules?
Colchicine is a drug that binds to free tubulin subunits, preventing them from assembling into microtubules.
This leads to microtubule disassembly, causing the cytoskeleton to break down.
Scientists use colchicine in experiments to study how microtubules form and function.
What is the Centrosome Made Of?
Centrosome Matrix
A fibrous structure that holds together more than 50 copies of γ-TuRC (γ-tubulin ring complex).
This γ-TuRC acts as a nucleation site for microtubule growth, making microtubule assembly faster and more efficient.
Centrioles
The centrosome contains two centrioles, which are cylindrical structures made of modified microtubules.
They are arranged in an L-shape (perpendicular to each other).
Accessory Proteins
The centrioles are surrounded by a variety of proteins that help regulate microtubule assembly, anchoring, and organization.
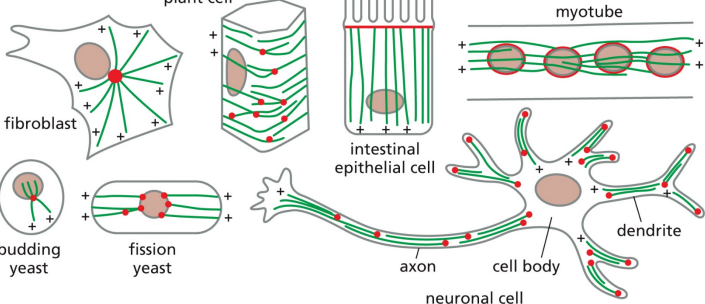
Microtubule organization varies among cell types
In fibroblasts, microtubules grow outward from a centrosome near the nucleus In neurons / intestinal cells, the organization is different In fungi, etc, MTOCs are embedded in the nuclear envelope Plants / fungi lack centrosomes, but still nucleate microtubules by γTuRCs
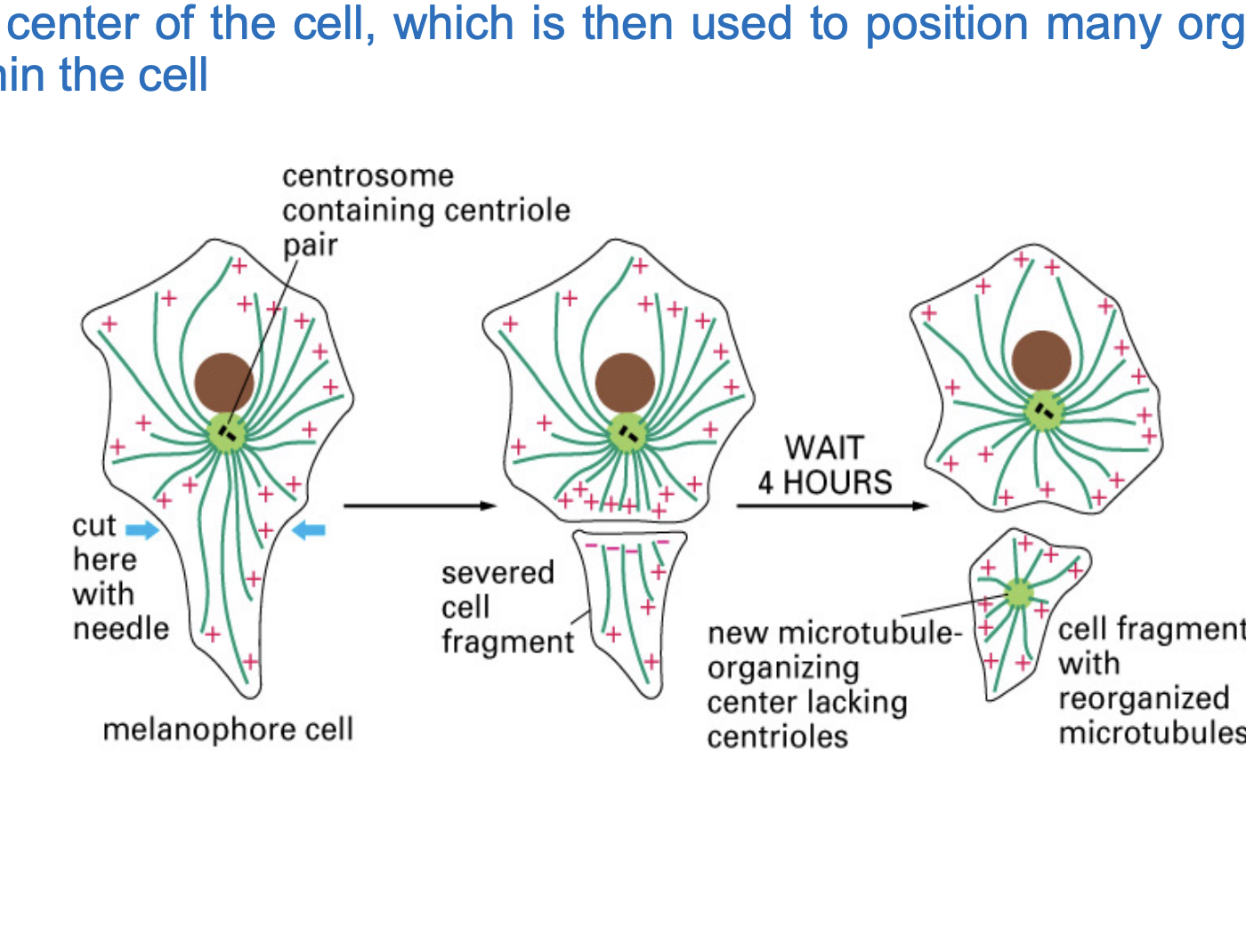
How Does the Centrosome Position Itself? (experiment)
Over time, microtubules began to grow from the centrosome.
As microtubules pushed against the edges of the well, the centrosome moved and positioned itself in the center.
microtubule network is self-organizing.
Inside the cell, this mechanism helps the centrosome stay in the middle
what happens to micrtubles during interphase?
Microtubules form a network across the cell, extending from the centrosome near the nucleus.
This helps with intracellular transport and maintaining cell shape
what happens to microtubules during Mitosis?
the centrosome duplicates and microtubules reorganize into a spindle-like structure.
This spindle attaches to chromosomes and pulls them apart to form two new cells.
How Do Centrosomes Duplicate?
The two centrioles separate from each other.
A new centriole grows perpendicular to each old centriole.
Microtubules begin to nucleate from each new centrosome, forming two organizing centers.
These two centrosomes move to opposite sides of the cell and form the mitotic spindle.
POORLY UNDERSTOOD!!
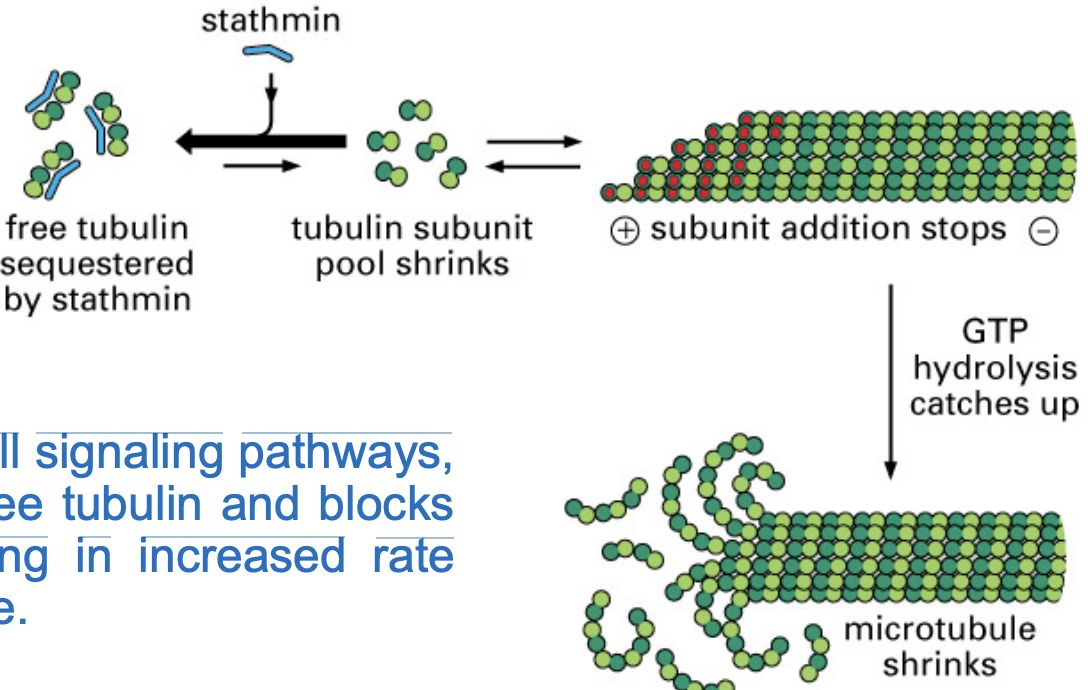
Stathmin
binds to free tubulin subunits, preventing them from assembling into microtubules.
MT catastrophe
this is a regulatory factor
Katanin
binds to the middle of a microtubule, not just the ends.
uses energy from ATP hydrolysis (breaking down ATP for energy).
This energy is used to sever (cut) the microtubule into pieces.
MAPs (Microtubule-associated proteins)
proteins that bind microtubules
regulate spacing, etc.
How Are MAPs Regulated?
controlled by phosphorylation & dephosphorylation by kinases
Phosphorylation → Usually reduces MAP binding to microtubules, making microtubules less stable.
Dephosphorylation → Restores MAP function, allowing them to stabilize microtubules again.
what are (+TIPs)
MAPs that specifically bind to plus ends of microtubles.
two main types of +TIPs
Stabilizers (Promote Growth)
XMAP215 → Helps stabilize the plus end and promotes microtubule assembly, allowing it to grow longer.
Destabilizers (Promote Disassembly)
Kinesin-13 (Catastrophe Factor) → Actively breaks down microtubules by pulling apart tubulin subunits, increasing the rate of microtubule catastrophe (rapid disassembly).
What is EB1-GFP?
EB1 is a +TIP protein that binds specifically to the plus ends of growing microtubules.
GFP (Green Fluorescent Protein) is a protein that glows under a microscope when exposed to specific light.
By genetically linking GFP to EB1, researchers can see where microtubules are actively growing in live cells.
what is the cell cortex?
a place near the plasma membrane where actin filaments are usually located
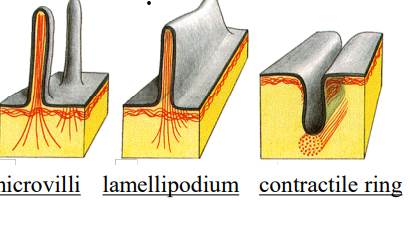
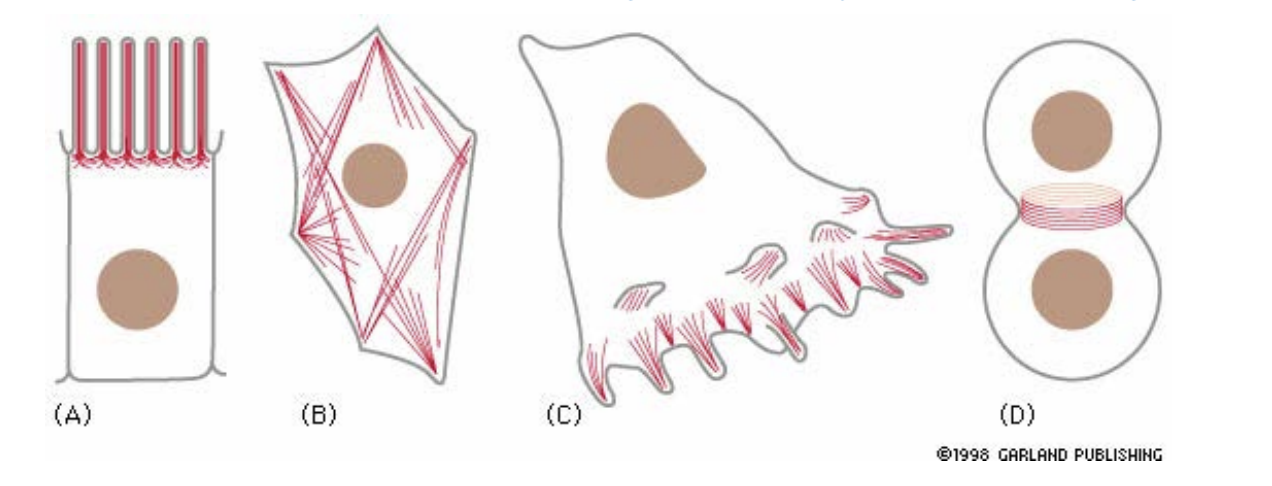
Types of actin filaments/ functions
A) Microvilli (increases absorption surface area in intestinal epithelial cells)
B) Stress fibers (adhesion to substrate)
C) Lamellipodia and filopodia (cell locomotion)
D) Contractile ring (pinches cells in two during mitosis)
E) Contractile apparatus of muscle cell
what are ARPs?
a complex nucleates actin filament growth from the minus end, allowing elongation at the plus end
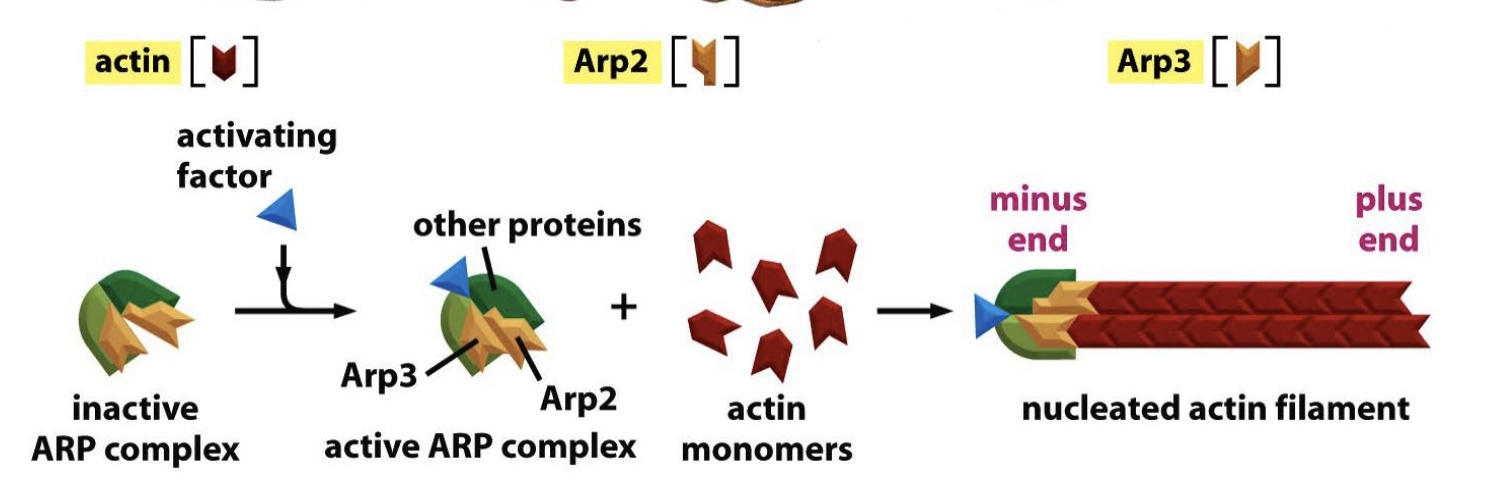
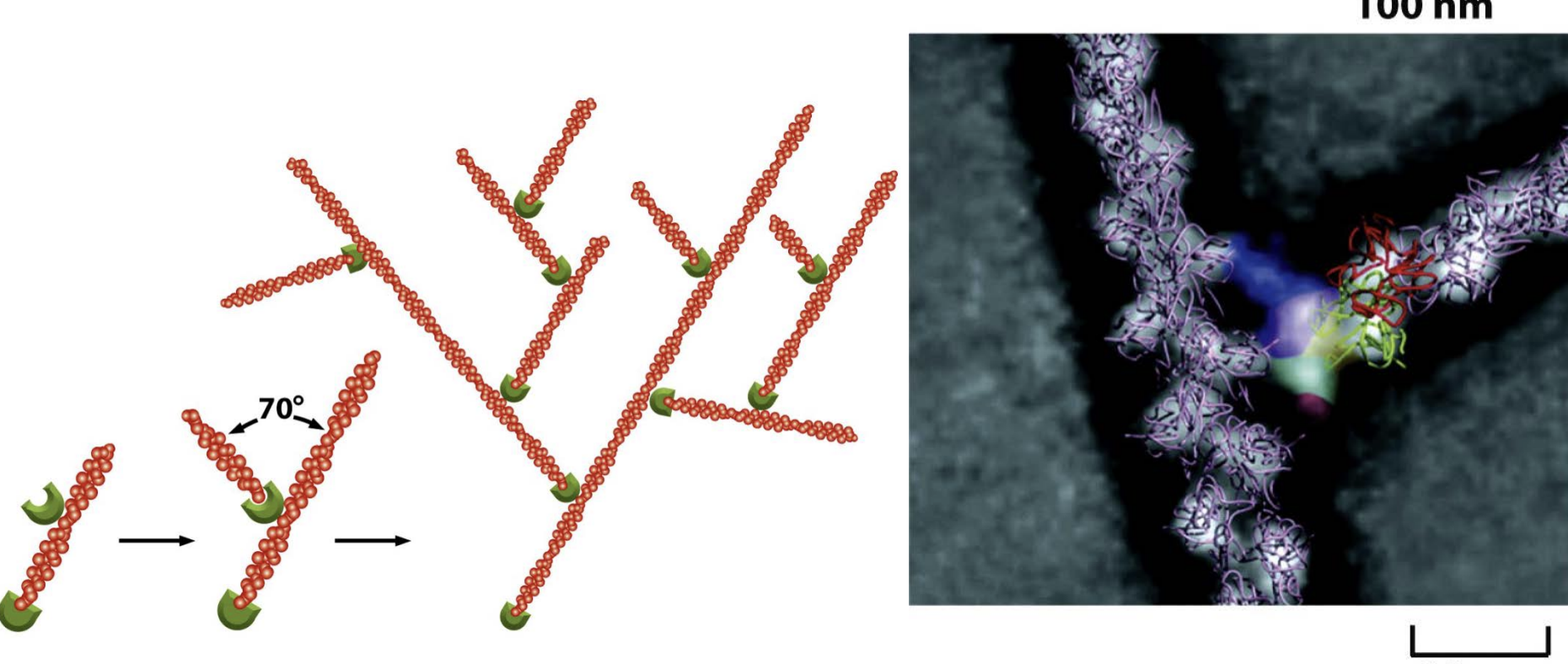
Actin web formation by the ARP (actin-related proteins) complex
starts the growth of a new actin filament at about a 70-degree angle from the original filament.
This branching pattern creates a dense, treelike network of actin filaments.
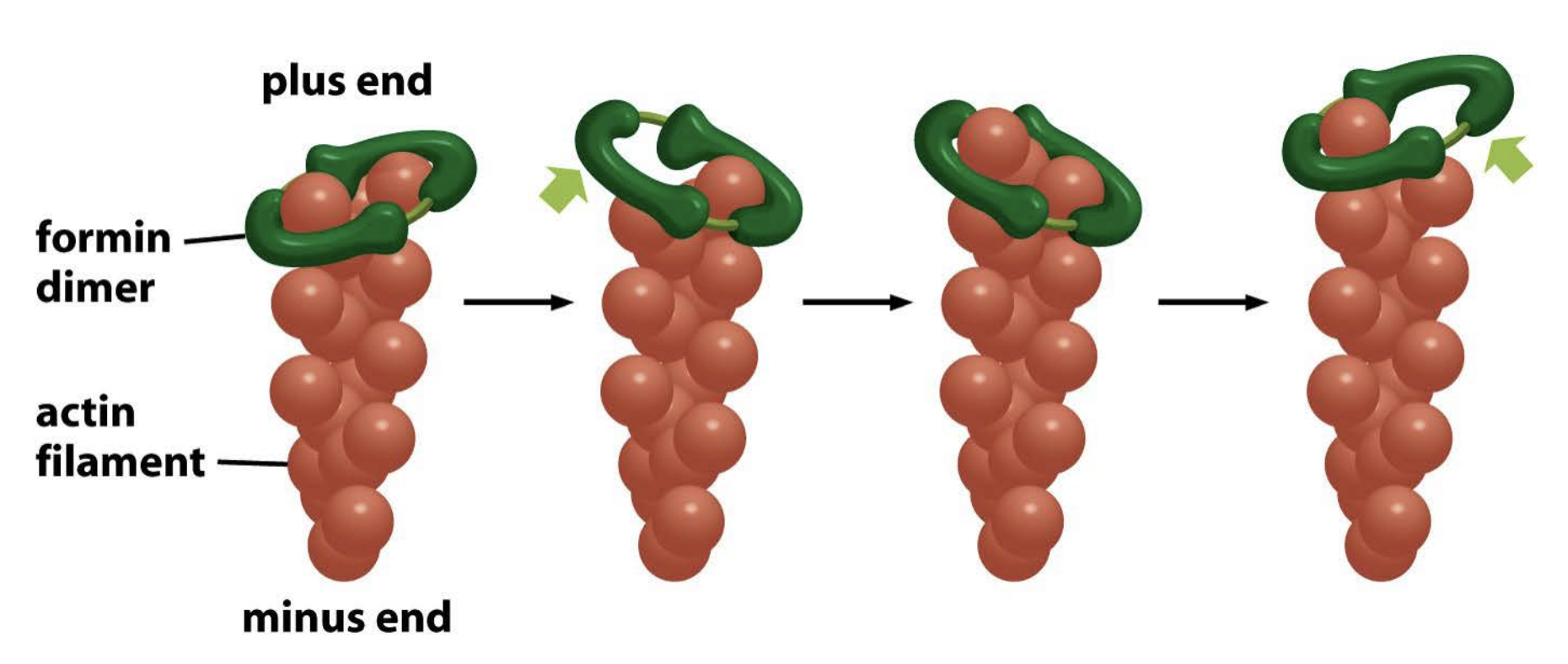
Formin
similar to ARP complex, but it is unbranched
both nucleates and accelerates actin filament growth.!!
self assembles into a dimer
and remains associated with the rapidly growing plus end as it elongates
Thymosin
an actin inhibitor by binding to the actin monomer preventing them from being added to the growing filament
profilin
promotes elongation and competes with thymosin by binding to actin monomers in a way that favors filament growth.
delivers actin monomers to the plus end, increasing the rate of polymerization
Thymosin and profilin activity is controlled by what?
signal transduction pathways
external signals like moving toward a stimulus (bacteria)
How Do Profilin and Formin Work Together?
Profilin Loads Actin Monomers for Growth
Formin Directs Elongation
Profilin Feeds Actin Monomers to Formin
what is something unique about actin filaments and their structure?
-they can assemble and arrange into several different types
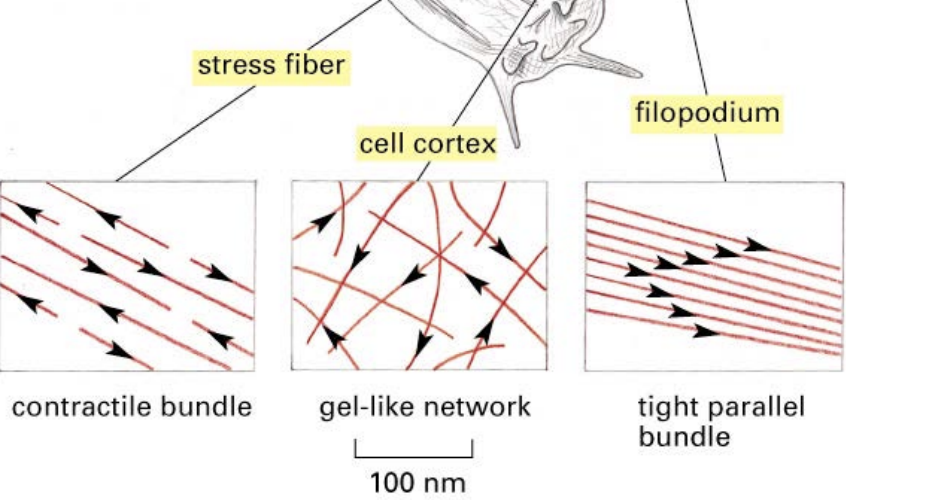
Four Key Actin-Crosslinking Proteins
fimbrin
a-actinin
filament
spectrin
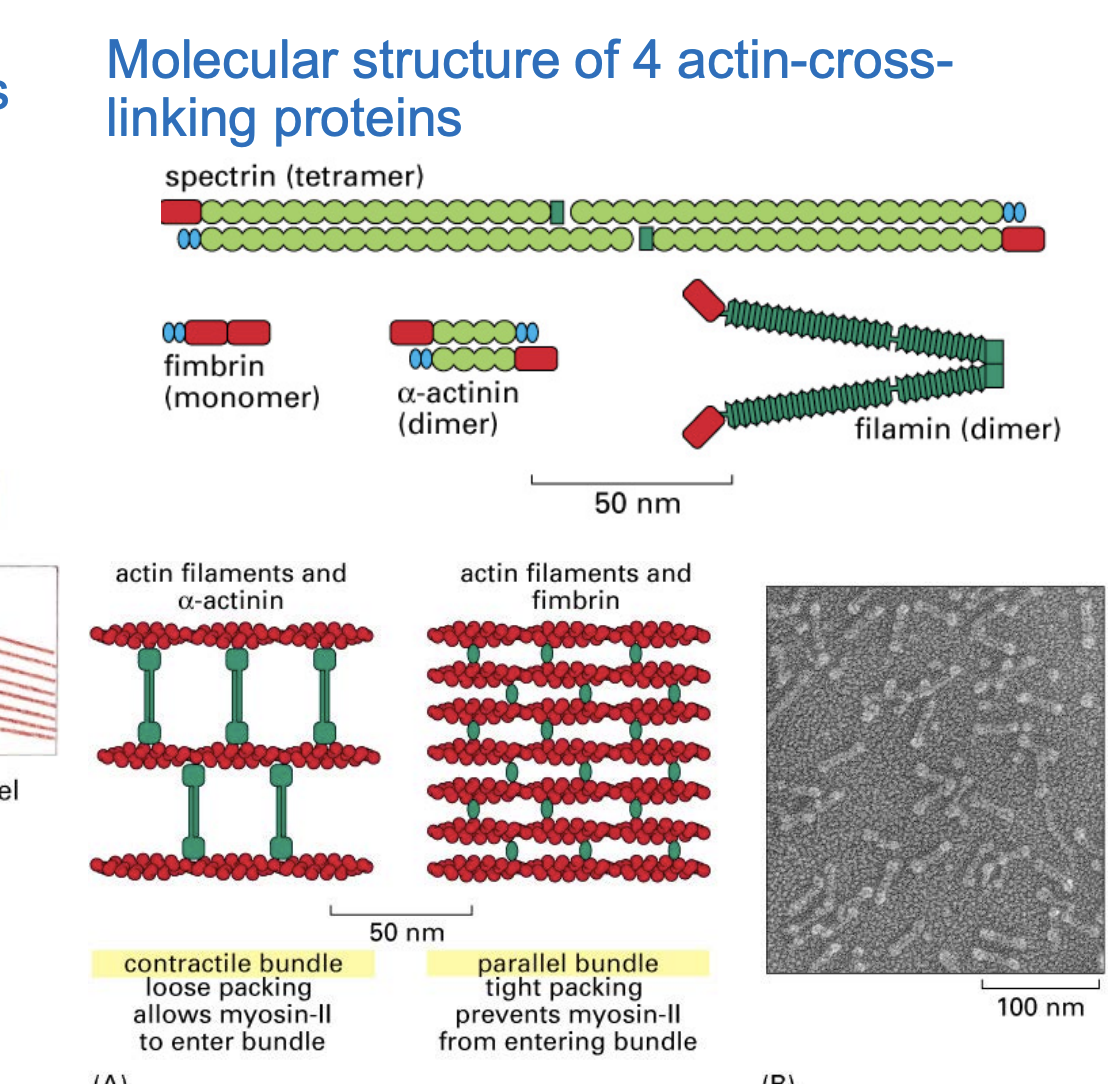
Fimbrin → Forms tight parallel bundles
actin ♰☩ † linking protein
Found in structures like filopodia and microvilli.
Helps create rigid, closely packed actin bundles.
α-Actinin → Forms loose, contractile bundles
+actin cross-linking protein+
Found in stress fibers and contractile rings (important for cell adhesion and division).
Allows myosin to enter, enabling contraction.
Filamin → Forms gel-like networks
+actin cross-linking protein+
Crosslinks actin into a web-like structure (important for cell shape and elasticity).
Essential for lamellipodia, helping cells crawl and change shape.
connects actin filaments at different angles,
Spectrin → Supports the cell cortex
+actin cross-linking protein+
Forms a strong, flexible network beneath the plasma membrane.
Essential for red blood cell structure and maintaining cell shape.
Actin filaments and cell shapes by microvilli
Found in intestinal epithelial cells to boost nutrient absorption.
Actin filaments form stiff, finger-like projections to maintain shape and stability.
Melanoma Cells with Low Filamin (Deficient in Filamin):
These cells struggle to form lamellipodia (the actin-based structures needed for movement).
As a result, they crawl poorly and do not metastasize effectively.
This suggests that filamin is essential for cell motility and cancer metastasis
Melanoma Cells with Restored Filamin Expression:
When scientists artificially restored filamin, the cells became highly metastatic.
The reintroduction of filamin enabled the cells to form lamellipodia and move efficiently, promoting metastasis (the ability of cancer to spread).
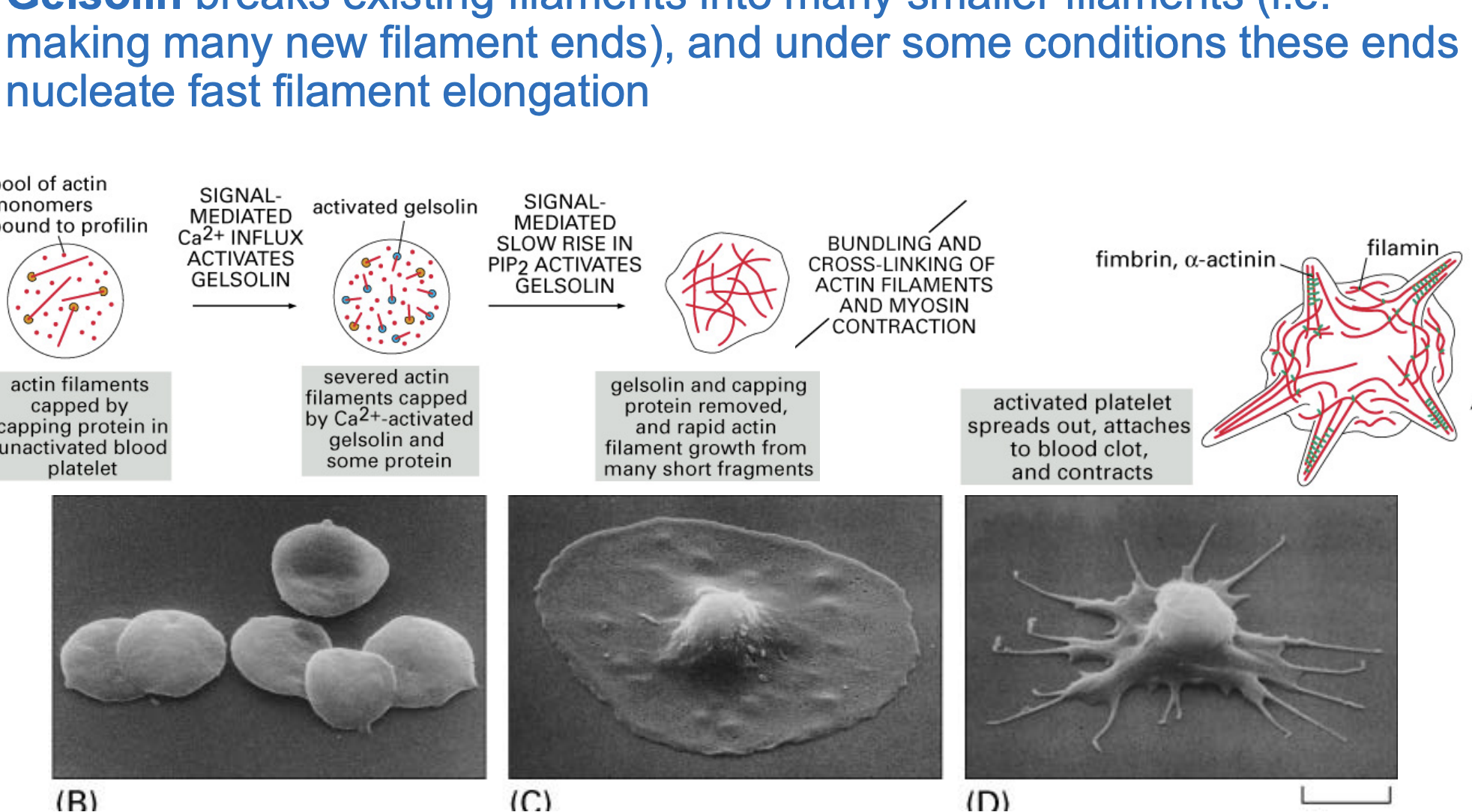
Gelsolins role in actin filament regulation and platelet activation
Inactive State
Blood platelets have actin filaments capped by proteins ( profilin), preventing uncontrolled growth.
Activation by Calcium (Ca²⁺) & PIP2
Gelsolin is activated by a Ca²⁺ influx, causing it to sever actin filaments into smaller fragments.
Rapid Actin Growth
Capping proteins are removed, and new actin filaments rapidly elongate from the severed fragments.
Platelet Shape Change
Actin filaments bundle and cross-link with myosin, leading to cell contraction.
Platelet spreads out and attaches to a blood clot, helping in wound healing and clot stabilization.
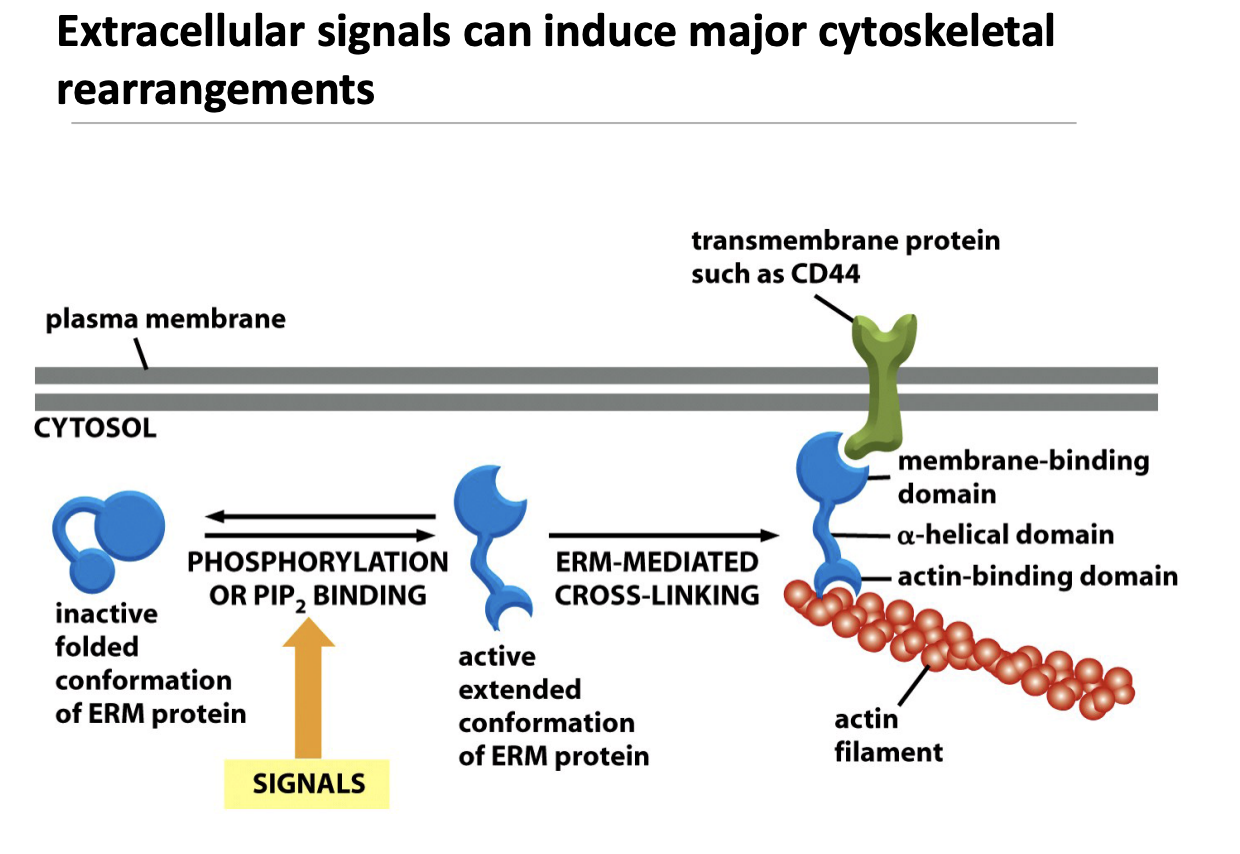
ERM protein mechanism (actin filament )
How Does This Work?
Inactive ERM Protein
ERM (Ezrin-Radixin-Moesin) proteins are initially in a folded, inactive state.
Activation by Phosphorylation or PIP₂ Binding
Extracellular signals lead to phosphorylation or binding of PIP₂, unfolding ERM proteins into their active form.
ERM Cross-Linking Actin to the Membrane
The activated ERM protein binds to a transmembrane protein (like CD44).
It also attaches to actin filaments, linking the cytoskeleton to the plasma membrane.
Like little helper holder guys anchoring these actins to the periferal of the cell
Rho family of monomeric GTPases (Rho, Rac, Cdc42)
Signals that affect cell shape, polarity, motility, and adhesion function
has numerous target proteins that affect actin organization and dynamics
what “tracks” do motor proteins walk along?
Actin
What is myosin (motor protein) made of?
Heavy chains (2 big parts): These have a head (the motor) that generates force and a tail (a long twisted part) that helps with structure.
Light chains (4 small parts): Help regulate the activity of the motor.
How do myosin molecules group together?
The tails stick together to form a thick filament (like a bundle of ropes).
These thick filaments have hundreds of myosin heads sticking out in opposite directions.
Myosin I
Has one head (the part that moves).
The tail part can attach to either actin or membranes.
Helps with moving things around inside the cell (intracellular organization).
Myosin II
Has two heads (which work together).
The tail parts wrap around each other, forming a dimer (a two-part structure).
This type is involved in contraction, like in muscles.
Myosin II Filament
Many Myosin II proteins group together by their tails sticking to each other.
Phosphorylation of myosin II light chains results in bipolar filament assembly
myosin VI
very different from other myosins as it moves to the minus end of actin
step by step process of myosin movement
Myosin Head Starts Attached to Actin
At the beginning, the myosin head is tightly attached to the actin filament.
But it can’t move yet because it needs energy.
ATP Binds → Myosin Lets Go
When ATP binds to the myosin head, the head lets go of actin.
This is like releasing your grip on the rope before reaching forward.
ATP is Broken Down → Myosin Cocks Back
The myosin head breaks down ATP into ADP and phosphate (Pi), which stores energy in the head.
This causes the head to cock back (like pulling your arm back to prepare for the next pull).
Myosin Re-Attaches to Actin (Further Along the Filament)
The myosin head attaches again, but now a little further forward along the actin filament.
Power Stroke: Myosin Pulls Actin
When phosphate (Pi) is released, myosin changes shape and pulls the actin filament.
This is like pulling yourself forward on a rope.
ADP is Released → Cycle Repeats
After the pull, ADP is released, and the myosin is ready to grab another ATP to start the cycle again.
These motor proteins move along what filament?
Kinesins
Dyenins
Microtubules.
these motor proteins act like a highway transportation system, ensuring everything gets to the right place.
kinesins
move toward the plus ends of microtubules and deliver cargo to cell edges
cytoplasmic dyenins
move towards negative ends, moving it towards nucleus (center of cell)
Plays a crucial role in intracellular transport and cilia/flagella movement.
accessory proteins to attach to membrane-bound organelles.
MUCH larger than Kinesin and myosin
Ring-shaped head, related to hexameric ATPases
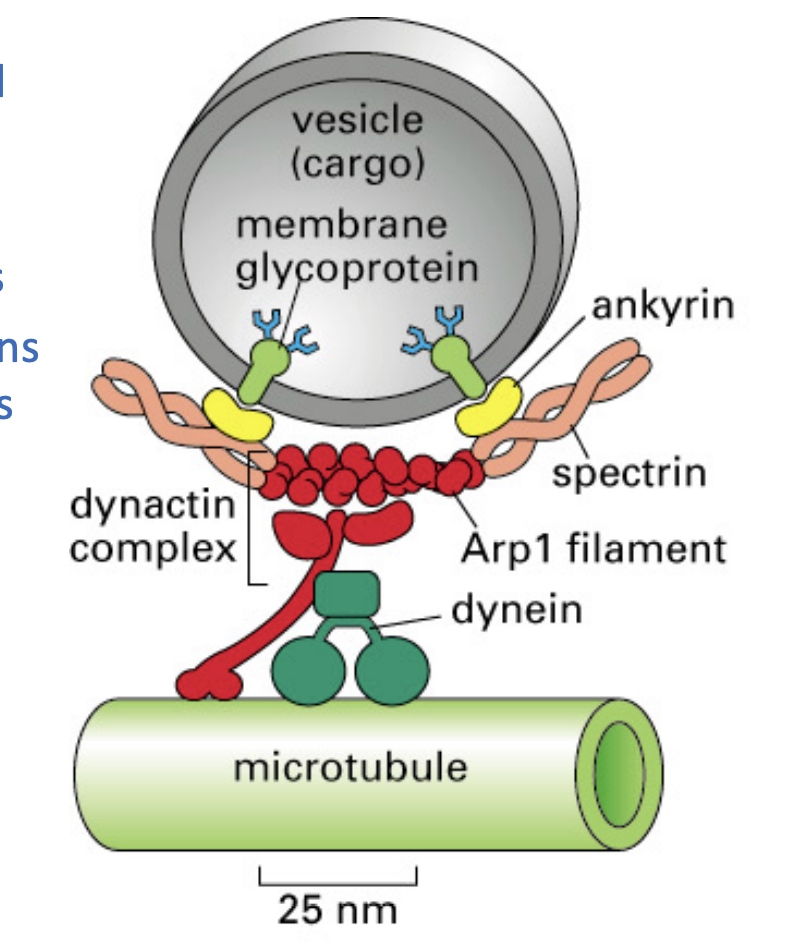
Myosin and kinesins are what?
like distant cousins and are structurally similar
both have motor domain ad chain like ropes
step by step mechanism of KINESIN movements
Starting Position: One Foot Forward, One Foot Back
Kinesin has two heads (like feet).
The rear (lagging) head is tightly bound to the microtubule because it has ATP attached.
The front (leading) head is loosely attached because it holds ADP instead of ATP.
Power Release: ATP Gets Used Up
The rear head (which had ATP) breaks ATP into ADP + Pi (hydrolysis).
This weakens its grip, so the rear head lets go of the microtubule.
Step Forward: ATP Switch Triggers Motion
The front (leading) head releases ADP and picks up a new ATP molecule.
This triggers a shape change in the “neck linker” (the flexible connection between the two heads).
The neck linker pulls the rear head forward, making it the new leading head.
Cycle Repeats: One Step at a Time
The new rear head will hydrolyze ATP and let go, while the new leading head binds ATP to take the next step.
This "hand-over-hand" motion lets kinesin move forward along the microtubule, pulling cargo like vesicles, organelles, or proteins.
Key Differences Between Kinesin & Myosin
feature | Kinesin 🚶♂ (Microtubules) | Myosin 🎯 (Actin Filaments) |
|---|---|---|
Motion Type | Step-by-step walking | Power stroke & release |
Always attached? | Yes, one head always on | No, detaches often |
Main Role | Cargo transport | Muscle contraction |
Cytoskeletal Track | Microtubules | Actin filaments |
ATP Use | ATP drives step movement | ATP powers grabbing & pulling |
Examples of Microtubule motor based transport
axonal transport
pigments granuls
organelle positioning
chromosome segregation
cilia/flagella movement
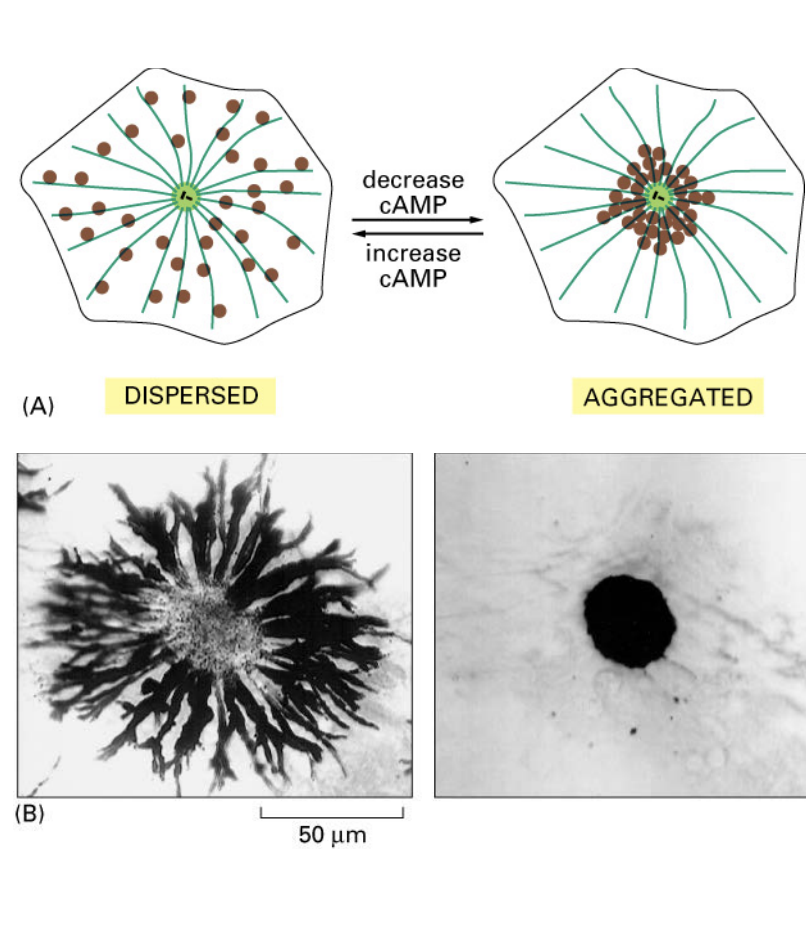
Regulated granule movement in melanocytes (fish pigment cells)
melanosomes can change their location within a cell in response to neuronal stimuli (cAMP levels)
kinesis disperses melanosomes toward cell edges (+ ends)
phosphate is added, inactivating kinesin and then dyenin takes over, pulling melanosomes near the nucleus (- end)
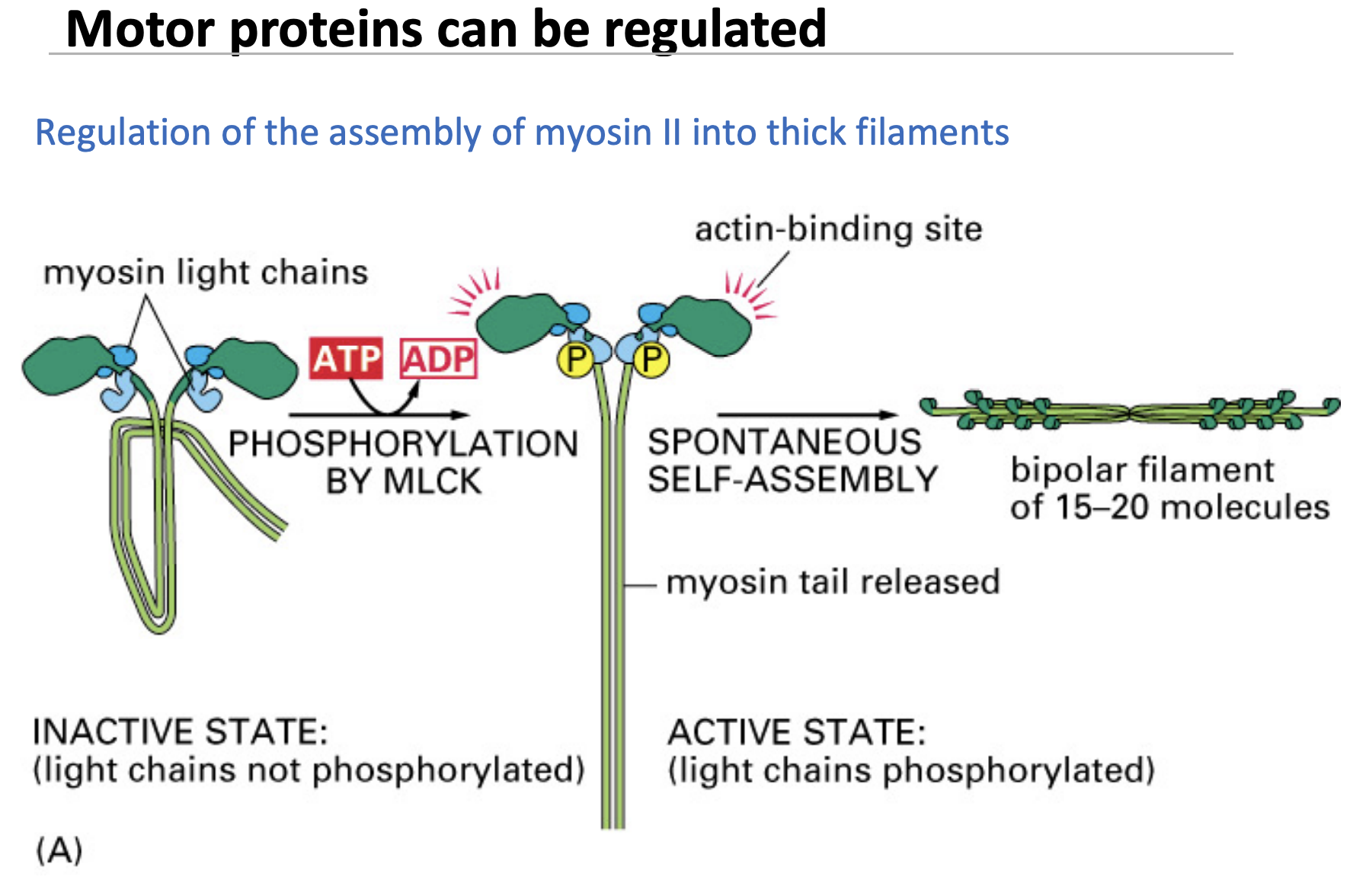
regulation of myosin assembly
Inactive myosin is folded and cannot function.
- Phosphorylation (by MLCK) unfolds myosin, allowing it to interact with actin.
Once activated, myosin self-assembles into thick filaments that drive contraction.
✅ Regulation ensures myosin is only active when needed, preventing unnecessary energy use.
what does muscle contraction depend on?
ATP-driven sliding of myosin II and actin filaments
running, walking—> skeletal muscle
♡ pumping and gut peristalsis—> cardiac muscle/smooth muscle
muscle cells
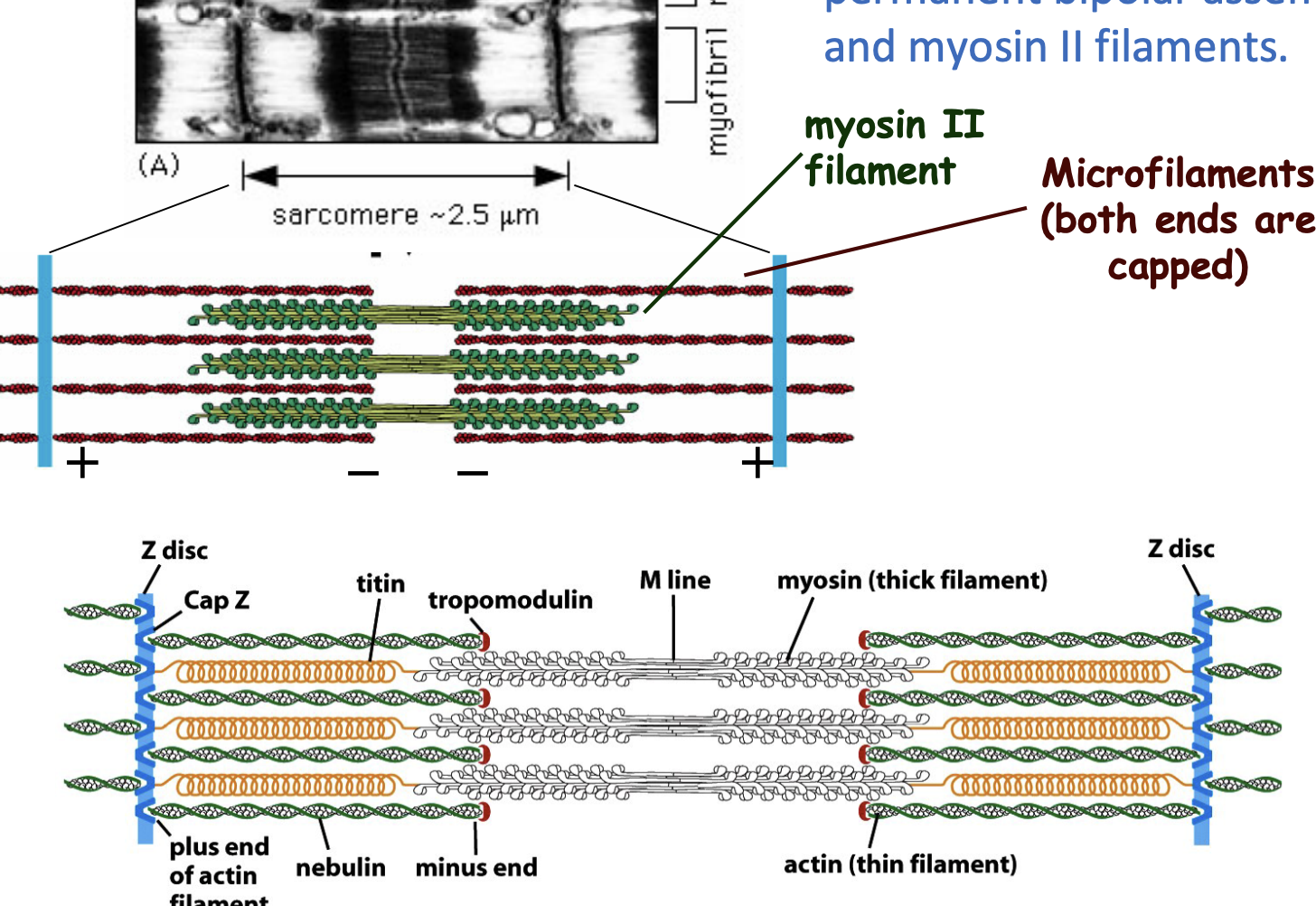
large, multi-nucleated cells that contain myofibrils
Myofibrils are made up of repeating units called sarcomeres, which are responsible for contraction.
contains permanent bipolar assemblies of actin and myosin II filaments.
organization of sacromeres
Actin (thin) filaments are attached to Z-discs, while myosin (thick) filaments pull on actin to generate force.
✅ Accessory proteins (Cap Z, Nebulin, Titin) help maintain structure and function.
✅ Contraction = Sarcomere shortens; Relaxation = Sarcomere lengthens.
how does myosin II aid in muscle contaction?
Think of a finger contracter things
pulls actin filaments, causing the sarcomere to shorten → Muscle contracts.
ATP is used to power myosin movement (ATP → ADP + Pi).
Each myosin thick filament has ~300 heads, and each head pulls actin 5 times per second.
Accessory proteins help keep everything organized and stable.
This process repeats rapidly to maintain muscle function.
Muscle Contraction & Calcium (Ca²⁺) Release (Simplified)
Muscle gets a signal from a nerve → This triggers an electrical impulse (action potential) in the muscle cell.
T-tubules (membrane folds) carry the signal deep into the muscle → They are in close contact with the sarcoplasmic reticulum (SR), which stores calcium (Ca²⁺).
Signal causes Ca²⁺ release from the SR → Calcium floods the muscle cell cytoplasm.
Ca²⁺ triggers muscle contraction → It allows myosin to interact with actin, shortening the sarcomere.
Ca²⁺ is pumped back into the SR (uses ATP) → This stops contraction and resets the muscle.
✅ Bottom line: Nerve signal → Ca²⁺ release → Muscle contracts → Ca²⁺ removed → Muscle relaxes.