Chem 1.11 Oxidation reduction reactions
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
oxidation
is a loss of electrons
reductions
gain in electrons
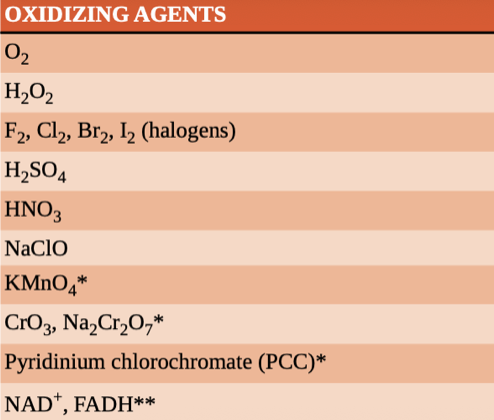
oxidising agent
facilitates the oxidation of another compound and is reduced itself in the process
Common oxidizing agents almost all contain oxygen or a similarly electronegative element.
eg (F, O, N, C)
electronegativity increases up the group and from left to right
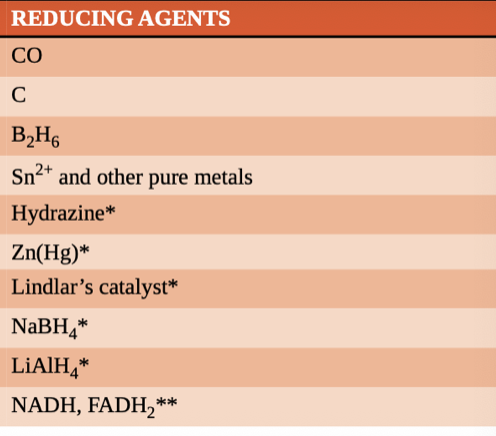
reducing agent
facilitates the reduction of another compound and is itself oxidized in the process
Oxidation numbers
are assigned to atoms in order to keep track of the redistribution of electrons during chemical reactions (to keep track of how many electrons are gained or lost)
(assumes unequal division of electrons in bonds "awarding" electrons to the more electronegative element)
Not the same as Formal charge (assumes unequal division of electrons in bonds, "awarding" one electron to each atom in the bond)
oxidation number of an atom in a compound is assigned according to the following rules:
The oxidation number of a free element is zero. For example, the atoms in N2, P4, S8, and He all have oxidation numbers of zero.
The oxidation number for a monatomic ion is equal to the charge of the ion. For example, the oxidation numbers for Na+, Cu2+, Fe3+, Cl–, and N3– are +1, +2, +3, –1, and –3, respectively.
The oxidation number of each Group I (1) A element in a compound is +1.
The oxidation number of each Group II (2)A element in a compound is +2.
The oxidation number of each Group VII (7)A element in a compound is –1, except when combined with an element of higher electronegativity. For example, in HCl, the oxidation number of Cl is –1; in HOCl, however, the oxidation number of Cl is +1.
The oxidation number of hydrogen is usually +1; however, its oxidation number is –1 in compounds with less electronegative elements (Groups IA and IIA). Hydrogen is +1 in HCl, but –1 in NaH.
In most compounds, the oxidation number of oxygen is –2. The two exceptions are peroxides (O2 2−), for which the charge on each oxygen is –1, and compounds with more electronegative elements, such as OF2, in which oxygen has a +2 charge.
The sum of the oxidation numbers of all the atoms present in a neutral compound is zero. The sum of the oxidation numbers of the atoms present in a polyatomic ion is equal to the charge of the ion. Thus, for (SO4 2−), the sum of the oxidation numbers must be –2.
The conventions of formula writing put cation first and anion second.
net ionic reactions
Half-reactions show the oxidation and reduction components of a redox reaction separately, while the net ionic equation shows only the species that directly participate in the overall reaction by eliminating "spectator ions
spectator ions
do not take part in overall reaction but simply remaining the solution unchanged.
rules for net ionic reactions
For reactions that contain no aqueous salts, the net ionic equation is generally the same as the overall balanced reaction.
For double displacement (metathesis) reactions that do not form a solid salt, there is no net ionic reaction because all ions remain in solution and do not change oxidation number.
Disproportionation (dismutation) reactions are a type of redox reaction in which one element is both oxidized and reduced, forming at least two molecules containing the element with different oxidation states.
Oxidation–reduction titrations are similar in methodology to acid–base titrations. These titrations follow transfer of charge.
Indicators used in such titrations change color when certain voltages of solutions are achieved
Potentiometric titration is a form of redox titration in which a voltmeter or external cell measures the electromotive force (emf) of a solution. No indicator is used, and the equivalence point is determined by a sharp change in voltage.
redox titration where no indicator is used. Instead, the electrical potential
difference (voltage) is measured using a voltmeter. As a redox titration progresses, its voltage changes; this is
analogous to following an acid–base titration with a pH meter instead of a color indicator