L7 Qualitative Molecular Orbital Theory Principles
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
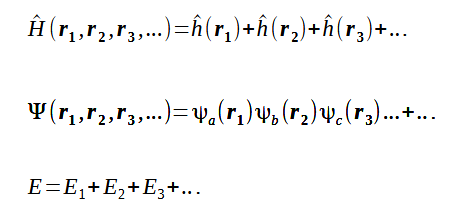
Approximations for electronic energies that ignore electronic repulsion are bad. How do we improve them
The orbital approximation includes electornic interactions within the Hamiltonian

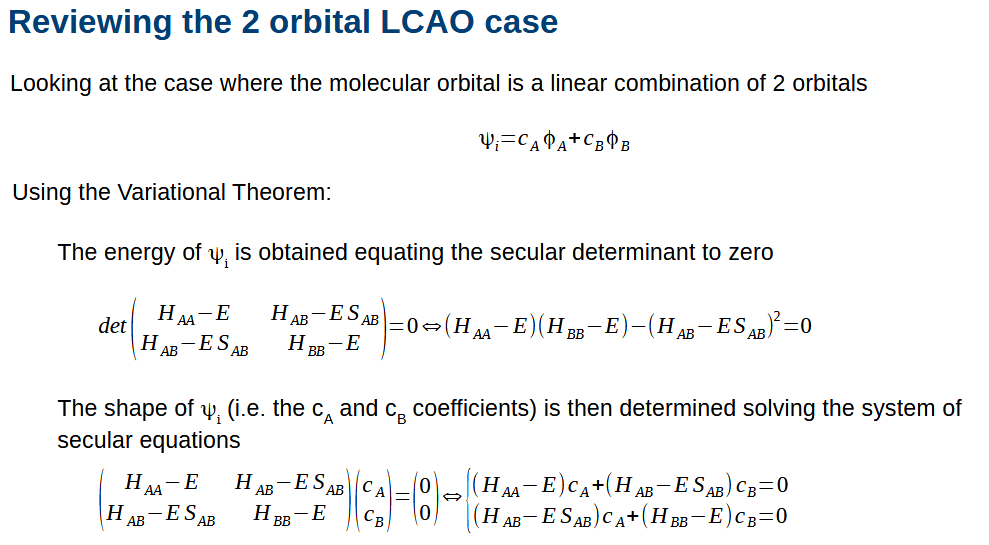
Using matrices for LCAO
Why does He2 not form
The stabilisation of the in phase combination is smaller than the out of phase destabilisation
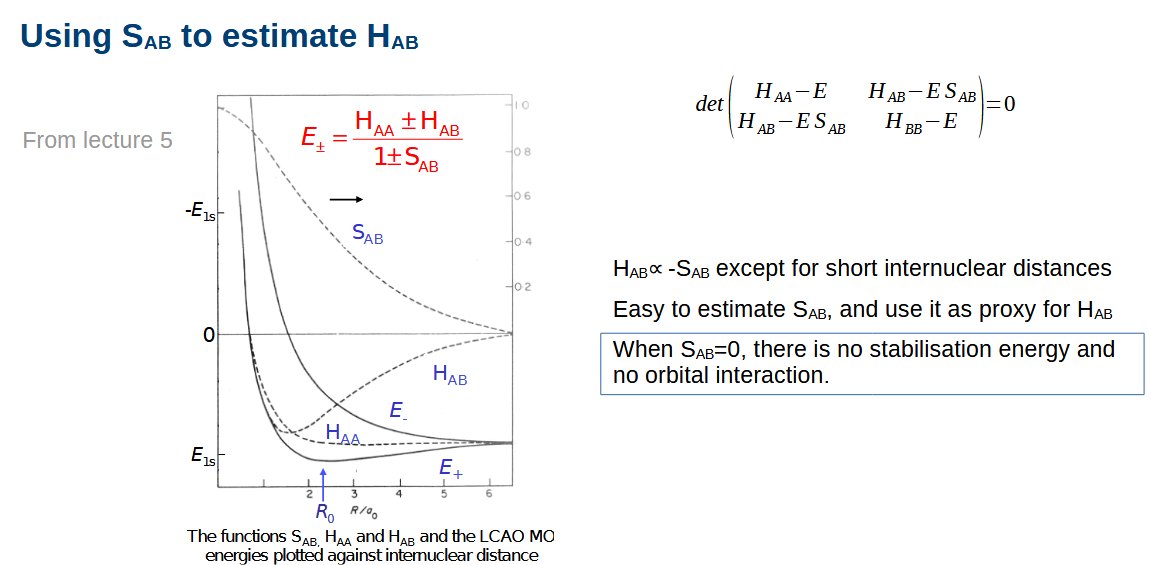
What is the overlap SAB and exchange integral HAB
Overlap - essentially just how much orbitals overlap. Is very small, is 1 at full overlap
The exchange integral is dependent on the overlap integral unless the internuclear distance is too small.
What does the size of an orbital drawn on an orbital mixing diagram mean
The degree of contribution cX with larger sizes meaning closer energy and more contribution. The electron density will mostly be on this side (think about how electronegative atoms have lower energy orbitals).
Why do atomic orbitals on the same atom not overlap (mathematically)
They involve multiplying S and AS which integrates to zero
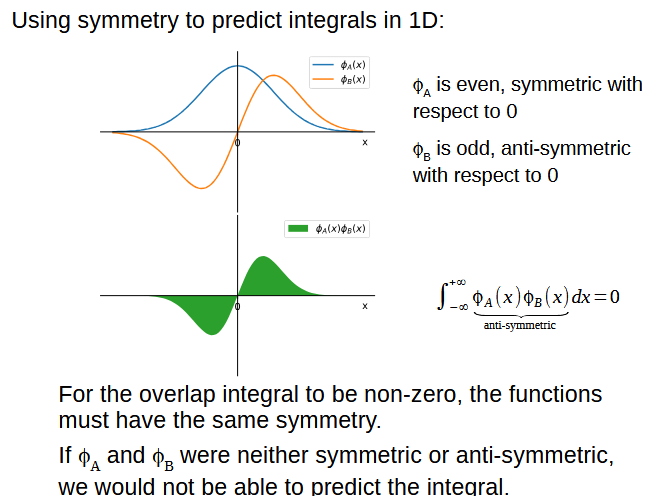
Why are core orbital interactions ignored
Core orbitals have much lower energy than valence orbitals and as such do not overlap enough to be considered
What symmetry needs to be fulfilled for orbitals to overlap
Their symmetry has to be the same
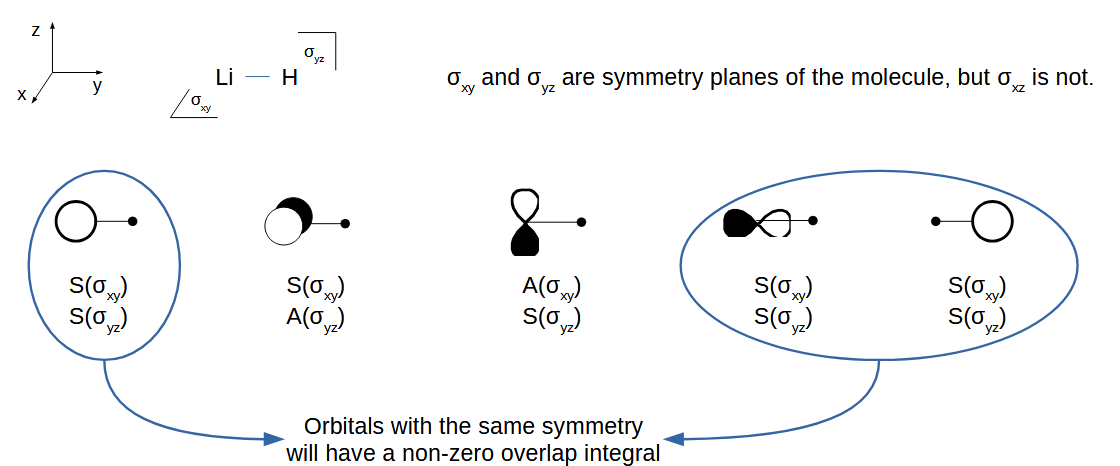
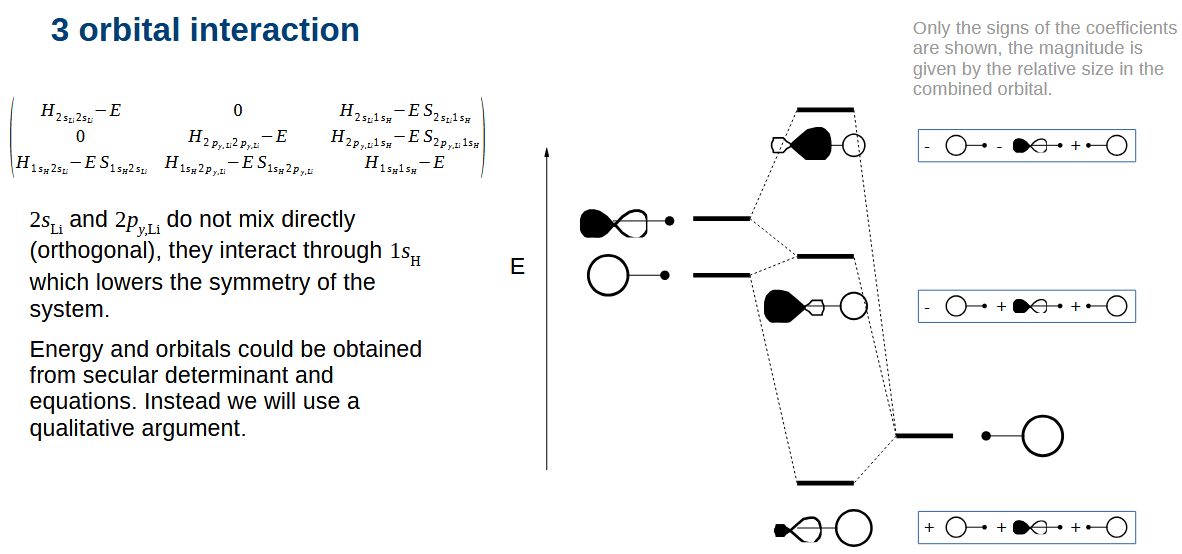
LiH orbital interactions diagram
The Li orbitals mix thanks to the H orbital. You can see that the combination at the bottom contains p character.