Drug design for the brain
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What structures are common in CNS active compounds?
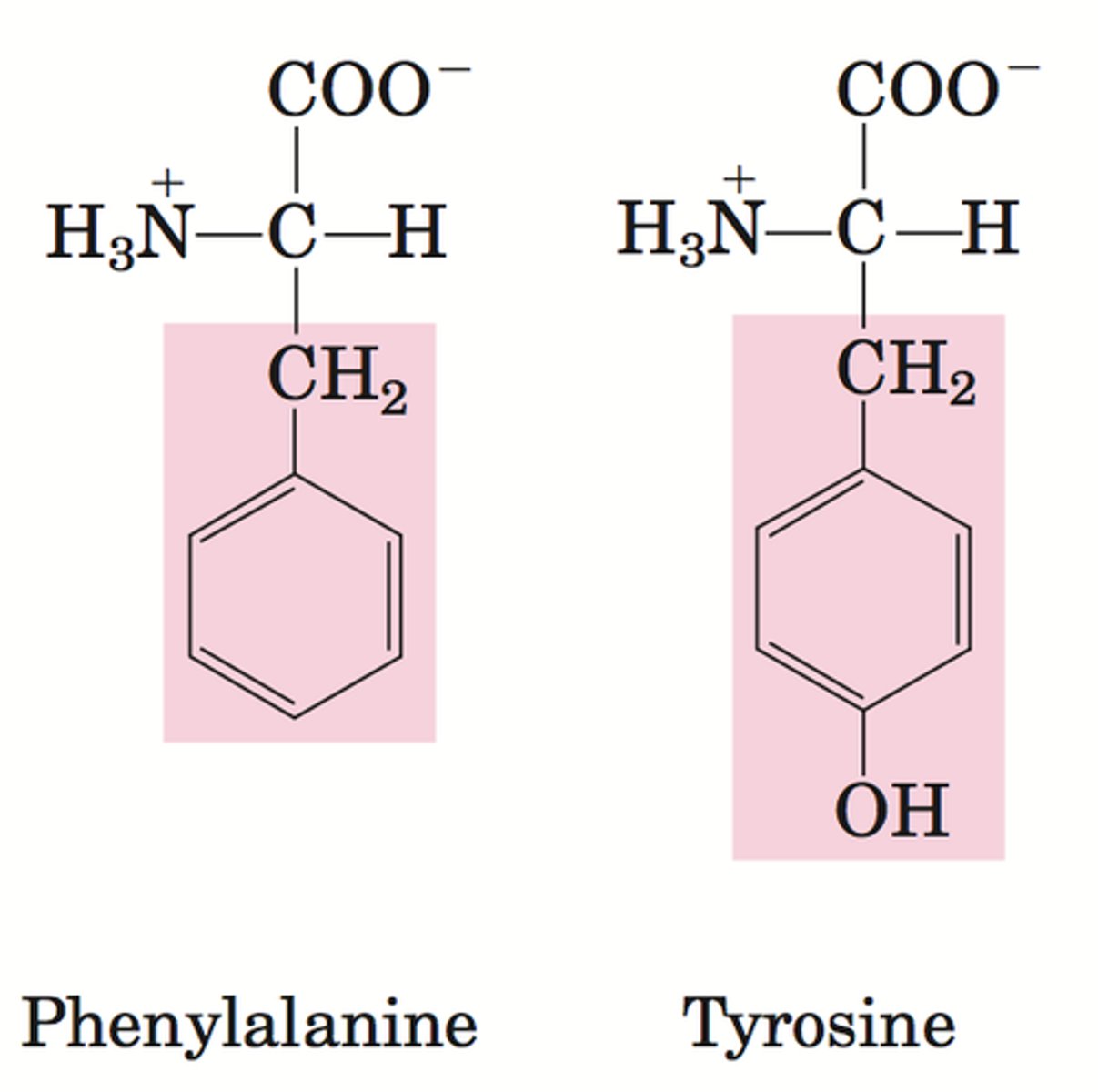
A phenylethylamine
This consists of
*an aromatic group
*a nitrogen 2 carbons away from the aromatic group.
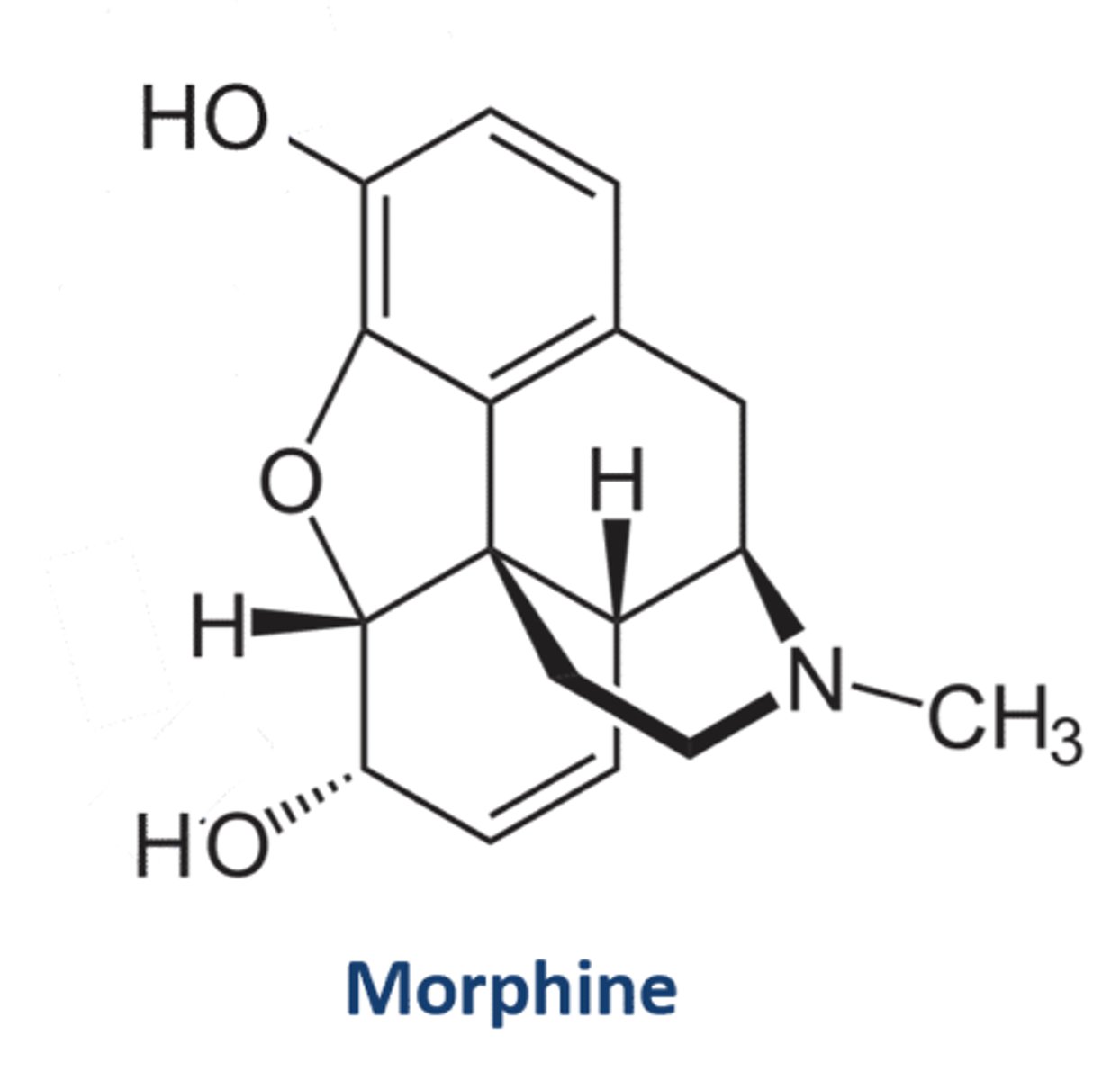
Here the structure of morphine is shown
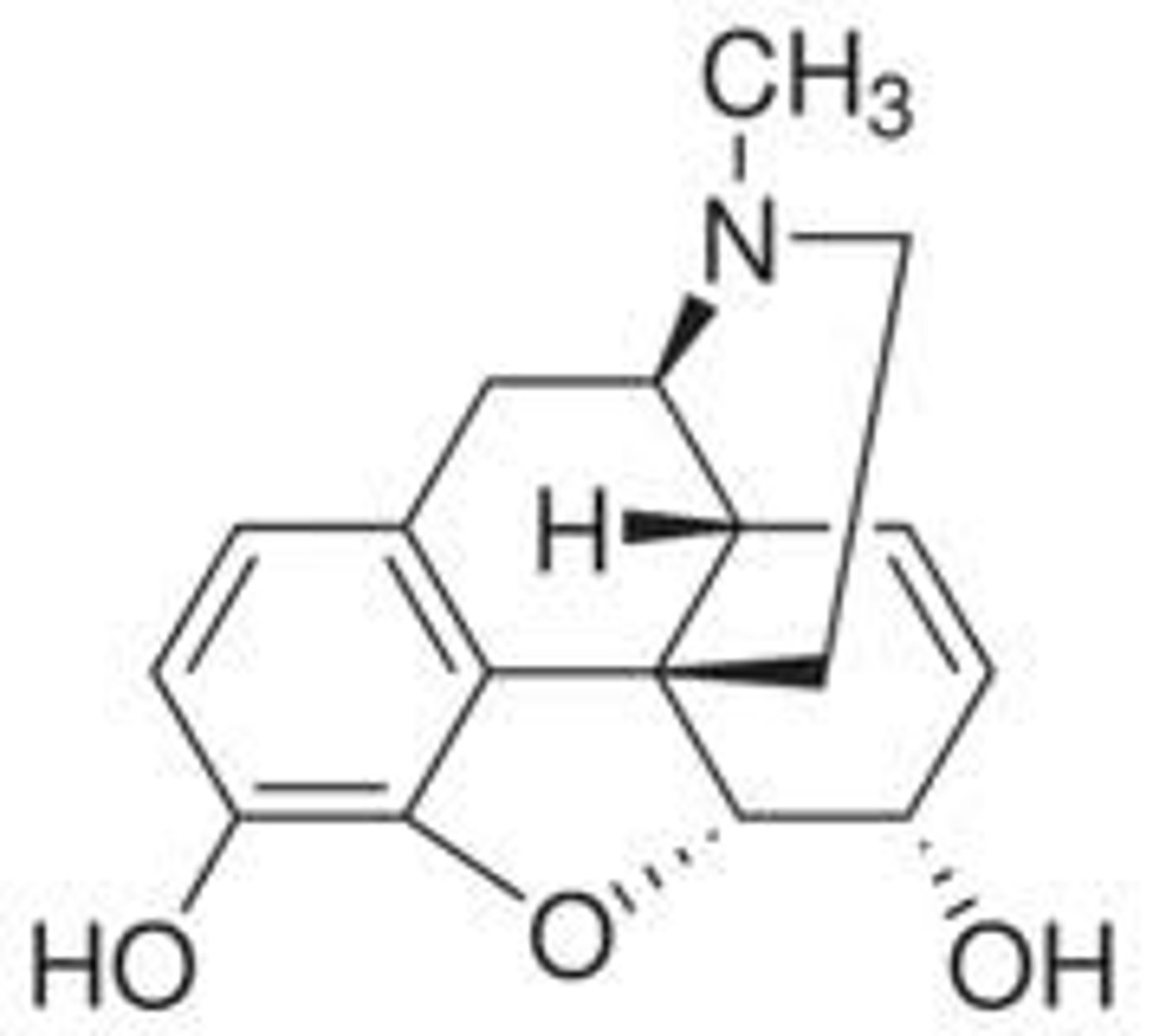
Why do many CNS active compounds contain a phenylethylamine unit or similar?
*The aromatic ring is lipophilic which is good for getting across the blood brain barrier (BBB) via transcellular transport/ passive diffusion.
*The nitrogen group seems to be linked to the activity of the drug once it enters the brain.
*Additionally, receptors in the brain interact with CNS natural products which are often derived from phenylalanine or tyrosine. Theses amino acids have an aromatic ring and a basic nitrogen two carbons. Hence why CNS active drugs mimic their structure.
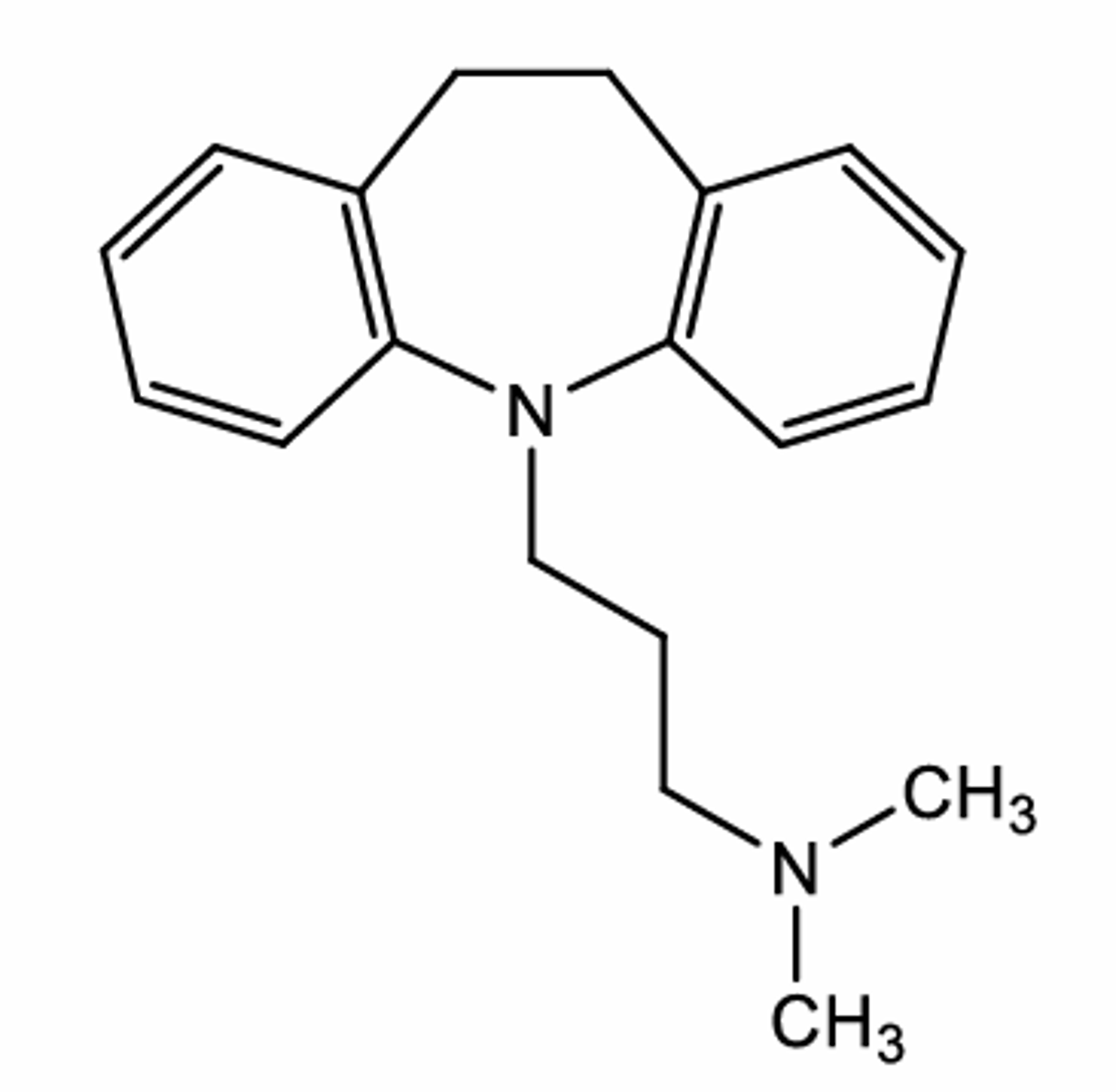
Some CNS active compounds do not have 2 carbons between the aromatic ring and the nitrogen atom. How may they still retain CNS activity?
There may be flexibility in the structure.
For example, here imipramine is shown which has more than 2 carbons between the nitrogen and the aromatic ring. The aliphatic chain separating the two groups is flexible meaning its bonds can rotate. So, spatially (3 dimensionally), the distance between the nitrogen and the carbon in imipramine may be similar to other CNS active drugs where the 2 groups are only carbons away.
What is the main way drugs cross the blood brain barrier?
Transcellular transport/ passive diffusion
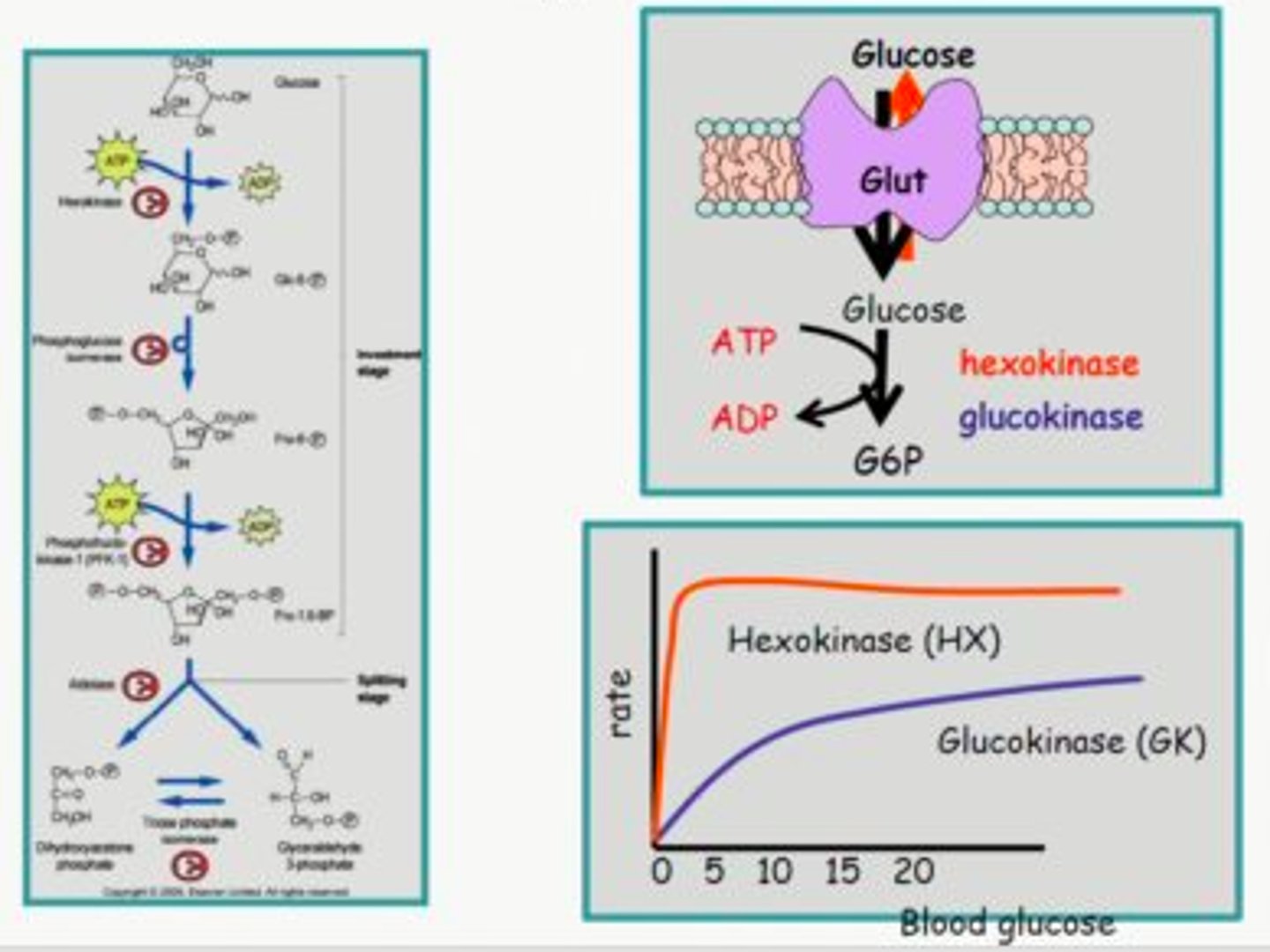
How do small non-polar molecules cross the blood brain barrier?
Passive diffusion/ transcellular transport
How do polar molecules cross the blood brain barrier?
Facilitated diffusion - the use of transporters.
This is only for polar compounds required for brain function such as glucose
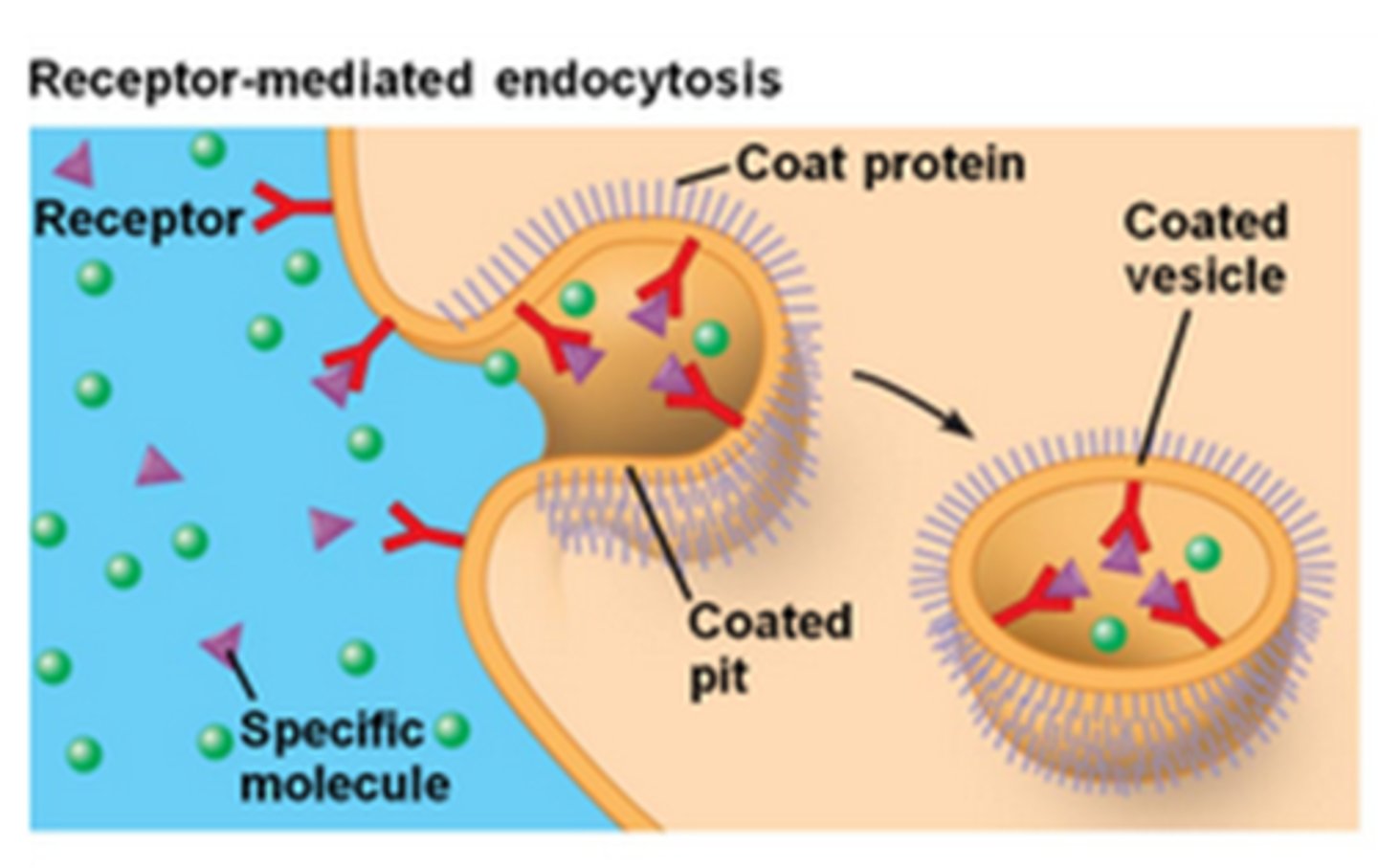
How do large molecules cross the blood brain barrier?
Receptor mediated transport
For large molecules such as insulin, they bind to the receptor and the whole structure is internalised in the cell. Then the large molecule is released.
What physiochemical properties must be considered for a drug to cross into the brain?
*The number of H-bonds (donor and acceptor)
*Lipophilicity
*Polar surface area (PSA)
*Molecular weight
*pKa (in particular, the acidity)
Compare Lipinski's guidelines for oral bioavailability to guidelines for crossing the blood brain barrier (BBB)
Both guidelines consider similar factors however guidelines for getting drugs into the brain tend to be slightly stricter.
*Lower amount of H bond donors
*Lower Mw
* Greater lipophilicity
* Higher pKa (so less acidic)
* Considers PSA
What is the PSA?
(Total) Polar surface area
The number of polar atoms (mainly nitrogen and oxygen) in a molecule and hydrogen atoms they are attached to.
What is the difference between LogP and LogD?
Both measure lipophilicity of a compound.
LogP is a single figure for lipophilicity; LogD considers the pH that the LogP was measured. This is because pH can change whether a compound it protonated or deprotonated (polar); hence it can change the lipophilicity as most polar compounds do not cross the BBB.
For both, a positive value indicates a more lipophilic compound and a negative value indicates a more hydrophilic compound.
Morphine structure
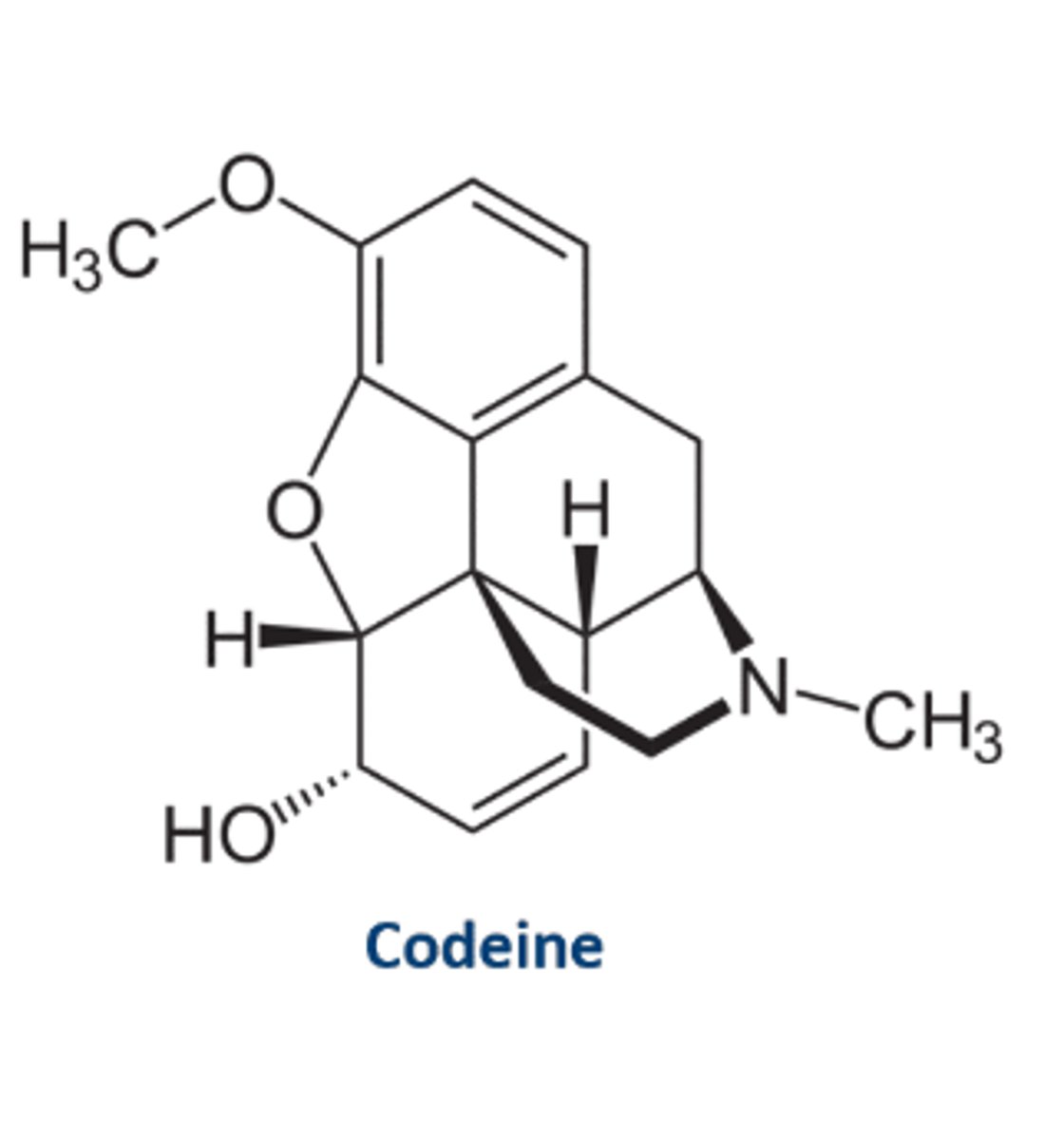
Codeine structure
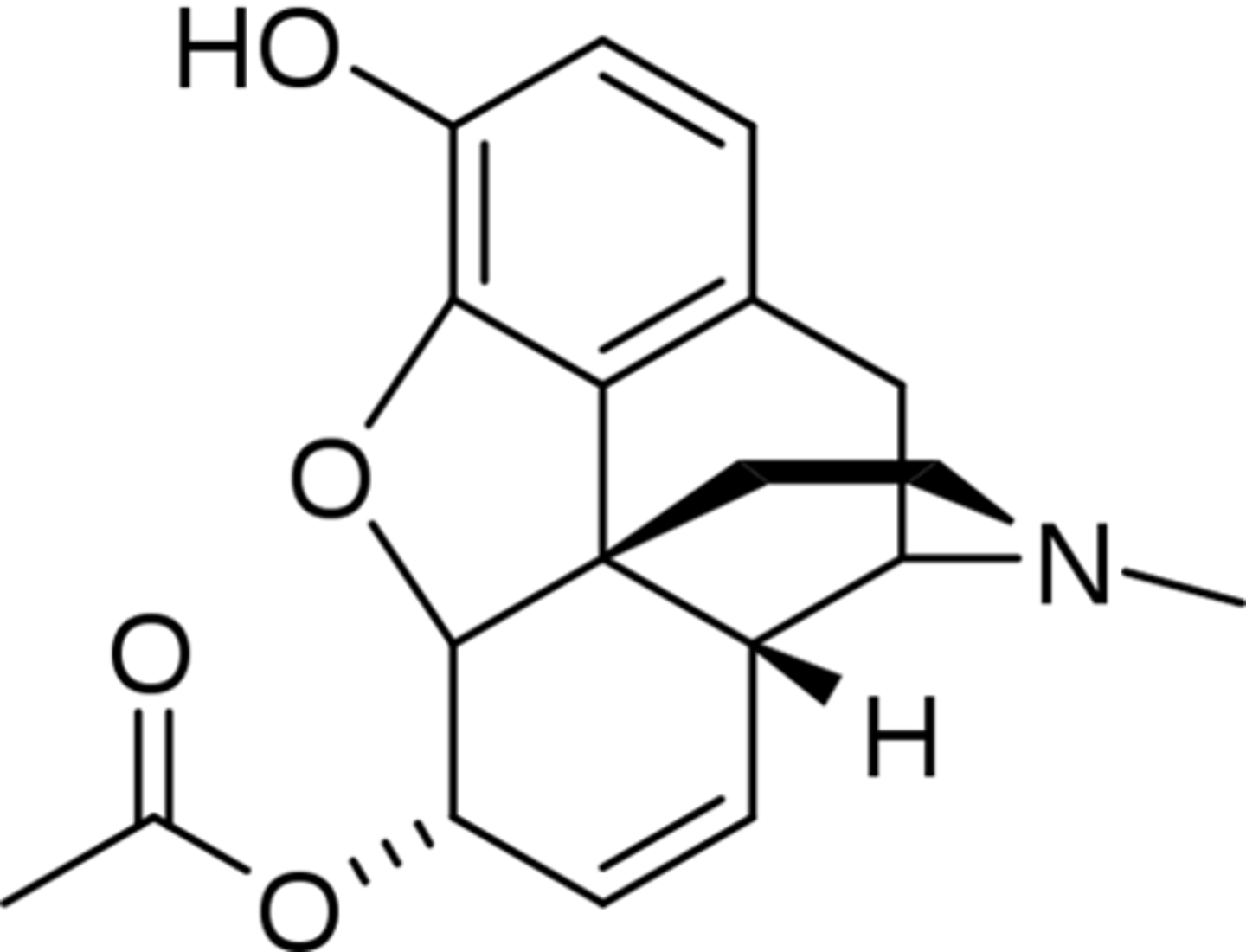
Heroine structure
What are Clark's (2003) guidelines for a compound which passes the blood brain barrier?
1. <6 polar atoms, ideally hydrogen bond donors less than 2
2. Polar surface area < 60-70 Armstrong
3. LogD 1-3. Positive value indicates a lipophilic compound.
4. ClogP (measure of lipophilicity) should be greater than the number of polar atoms. (summarised by ClogP - (N + O) > 0)
5. Mw <450
What are Partridge's (1998) guidelines for a compound which passes the blood brain barrier?
1. Hydrogen bonds (8-10)
2. Mw less than 400-500
3. No acids, not a lot of COOH which have reached the CNS
What are physiochemical properties which are good for transport across the blood brain barrier?
1. Lower molecular weight (around 400-500)
2. High lipophilicity
3. Low number of polar atoms (N and O) (particularly H-bond donors)
4. No carboxylic acids
Why is it recommended not to have a carboxylic acid in drugs which target the CNS?
Many CNS active drugs have an amine group. If you have an amine and a carboxylic acid then, no matter the pH, one of the groups will be charged. Charged molecules do not cross membrane.
Additionally, the amine group is needed as many CNS molecules rely on nitrogen for their desired effect once in the brain.
What does a high or low pKa indicate?
A high pKa = strong acid
A low pKa = weak acid
How may a polar drug cross the blood brain barrier?
Active transport using transporters
Give an example of a drug which is polar and crosses the blood brain barrier
Levodopa
Levodopa is basic and acidic (COOH and amine group). No matter what pH, it will always be charged - either amine protonated or carboxylic acid will be deprotonated. It cannot travel across the brain using passive diffusion.
Therefore, it must be actively transported into the CNS.
What are some more modern, specific physiochemical properties for making a drug which is likely to cross into the brain?
1. Low molecular weight: <400 Daltons
2. Low polar surface area: < 90
3. Moderate lipophilicity: logD between 1 and 3
4. Maximum of one H-bond donor
5. No acids
What ways can lipophilicity be increased in order to improve drug transport across the brain
Some ideas...
- Make OH into an ether
- Make COOH into aromatic (similar conjugation)
- Make COOH or OH into an ester