Functional Groups / Naming Organic Compounds
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
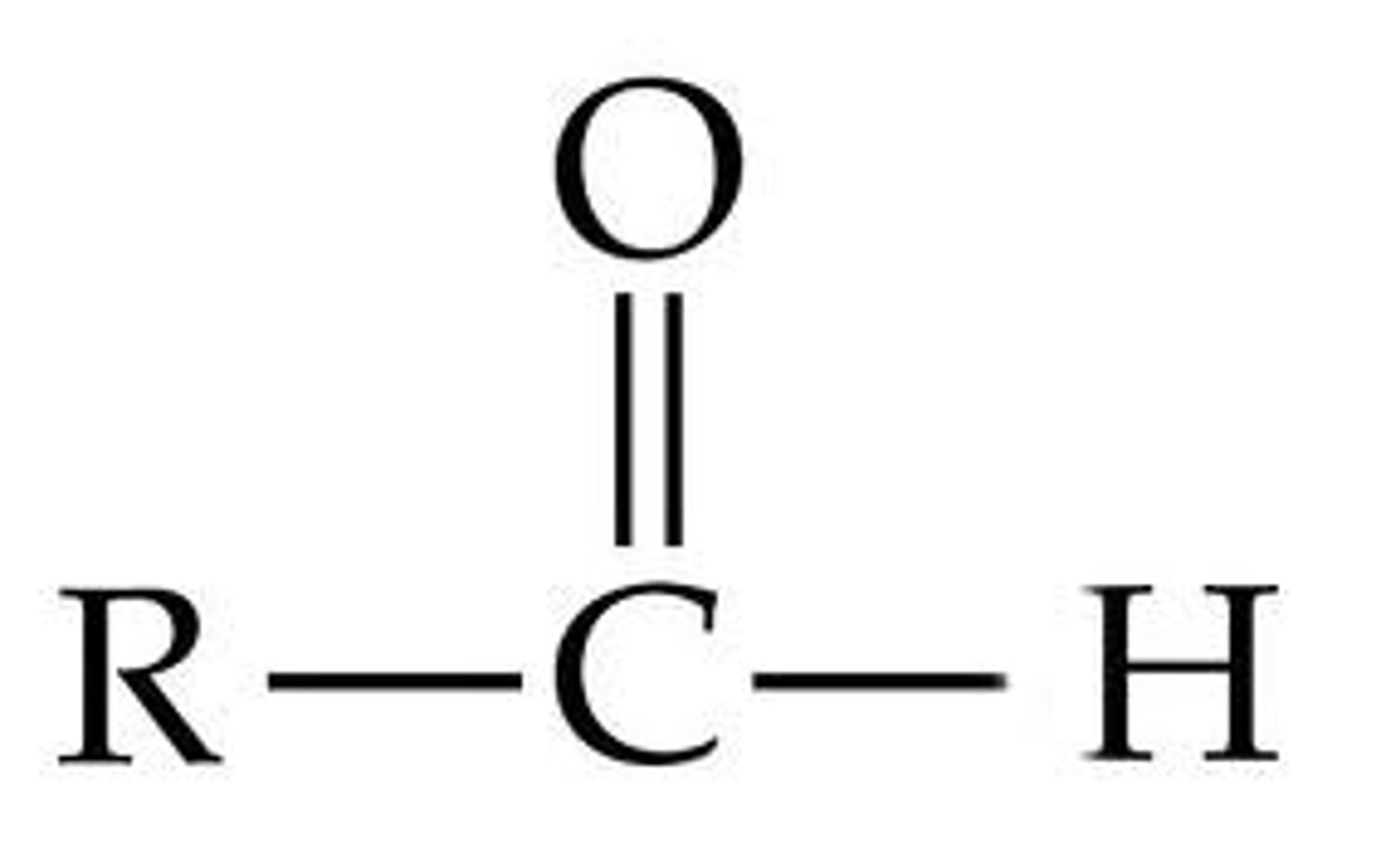
Ends in "-al"
Has one terminal carbonyl
Aldehyde
Aldehyde
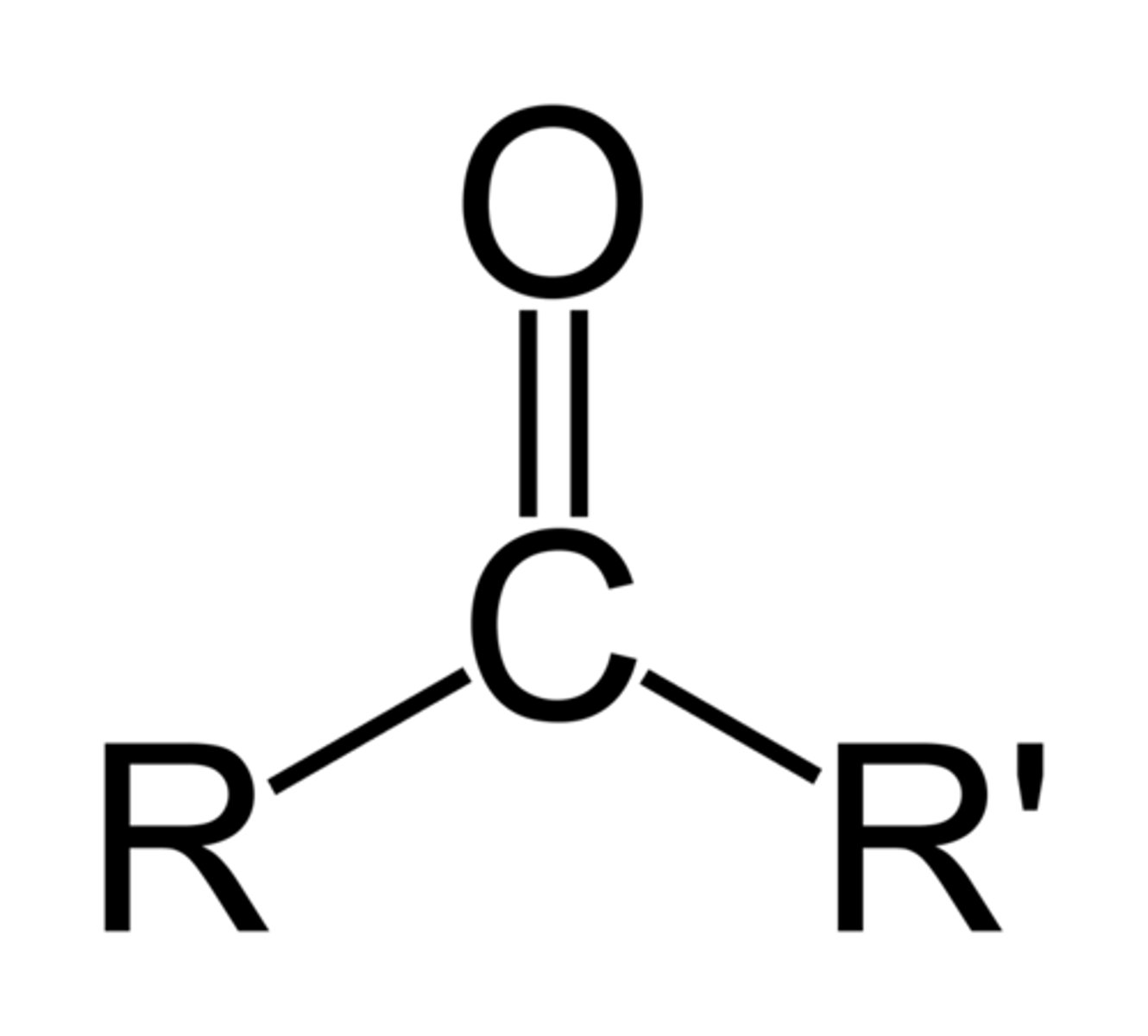
Ends in "-one"
Has one intermediate carbonyl
Ketone
Ketone
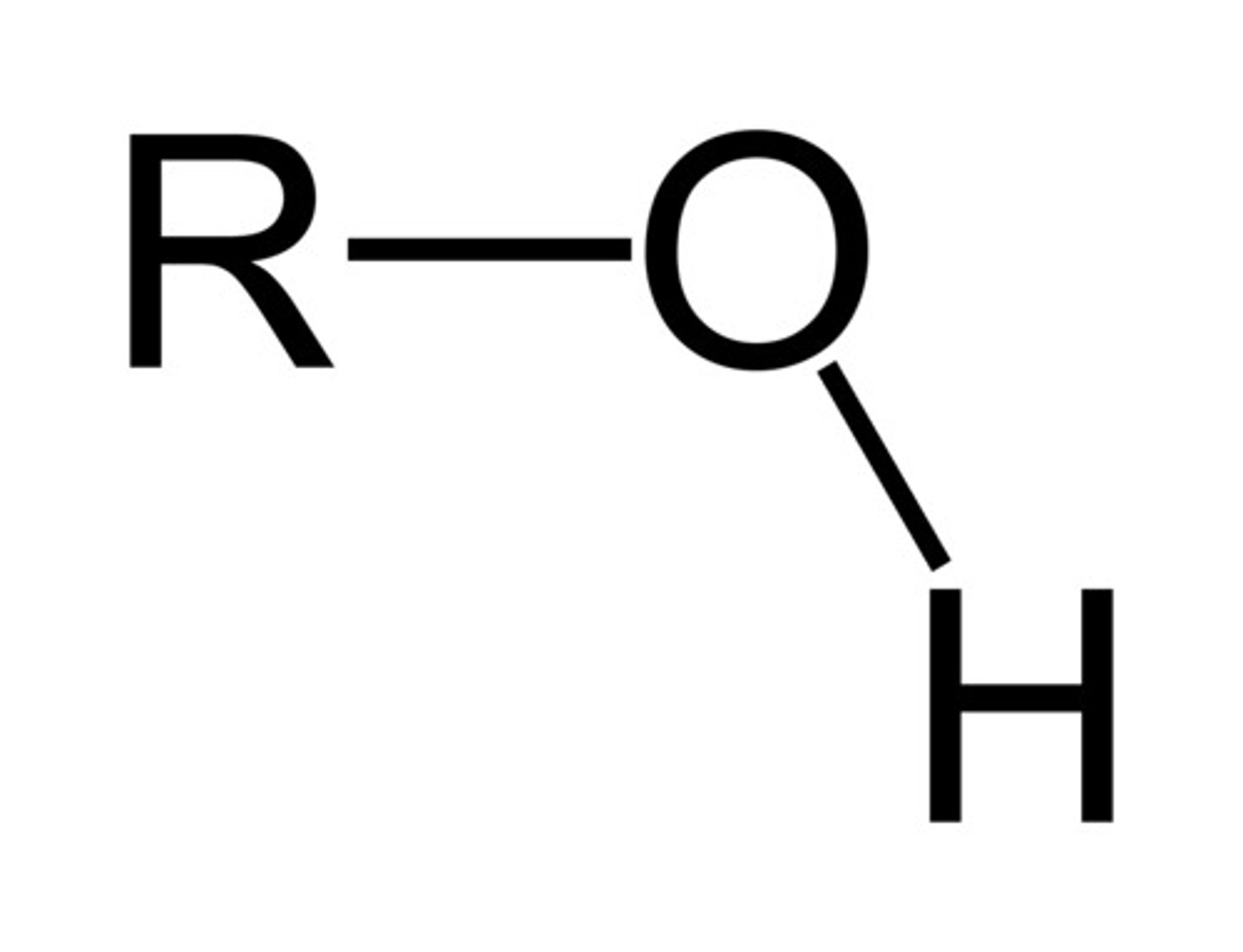
Ends in "-ol"
Begins with "hydroxy-" (if paired w/ other functional group that alters ending)
Has one hydroxide branch
Alcohol
Alcohol
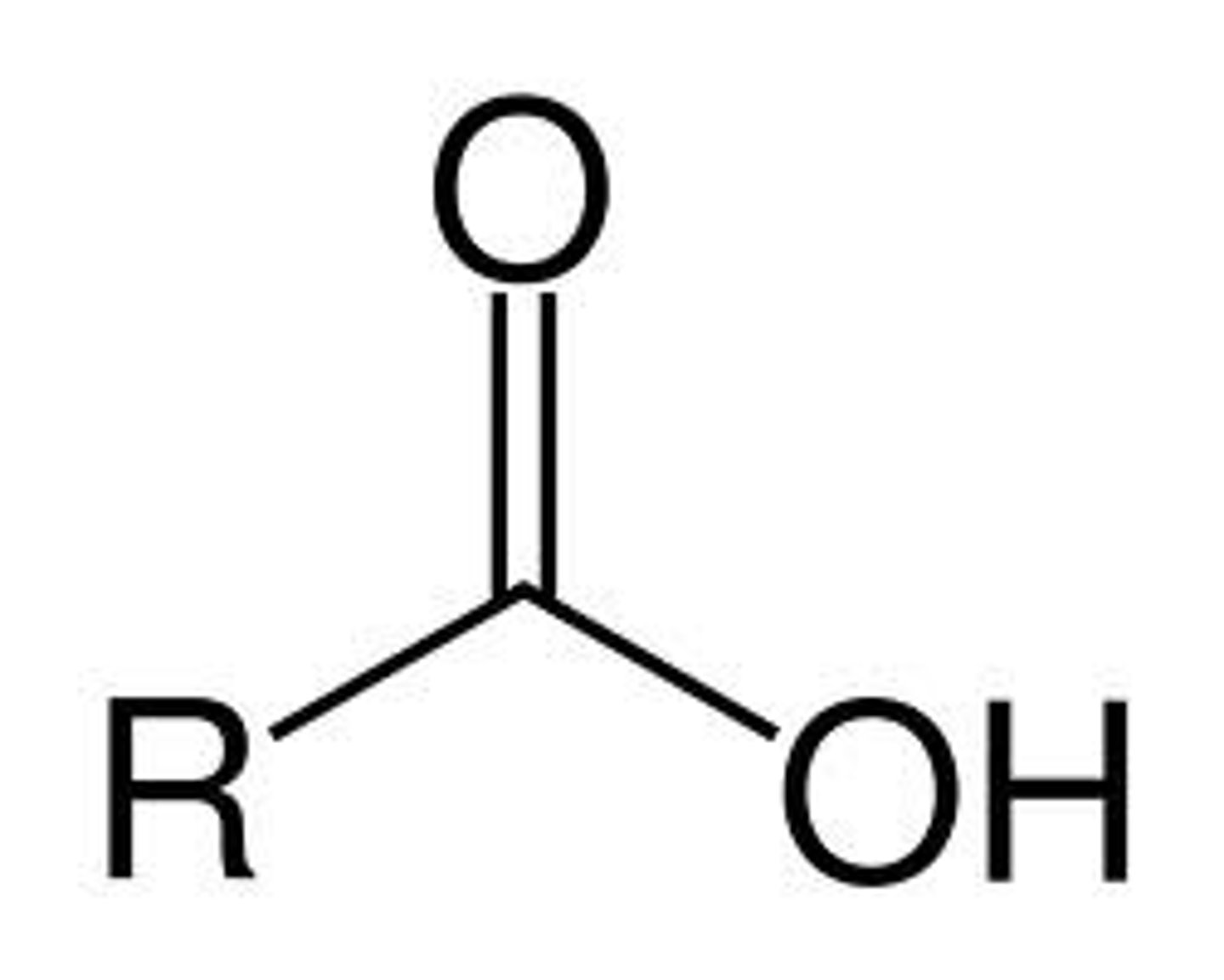
Ends in "-oic" + acid
Has a terminal carbonyl and hydroxide branch on the same carbon
Carboxylic Acid
Carboxylic Acid
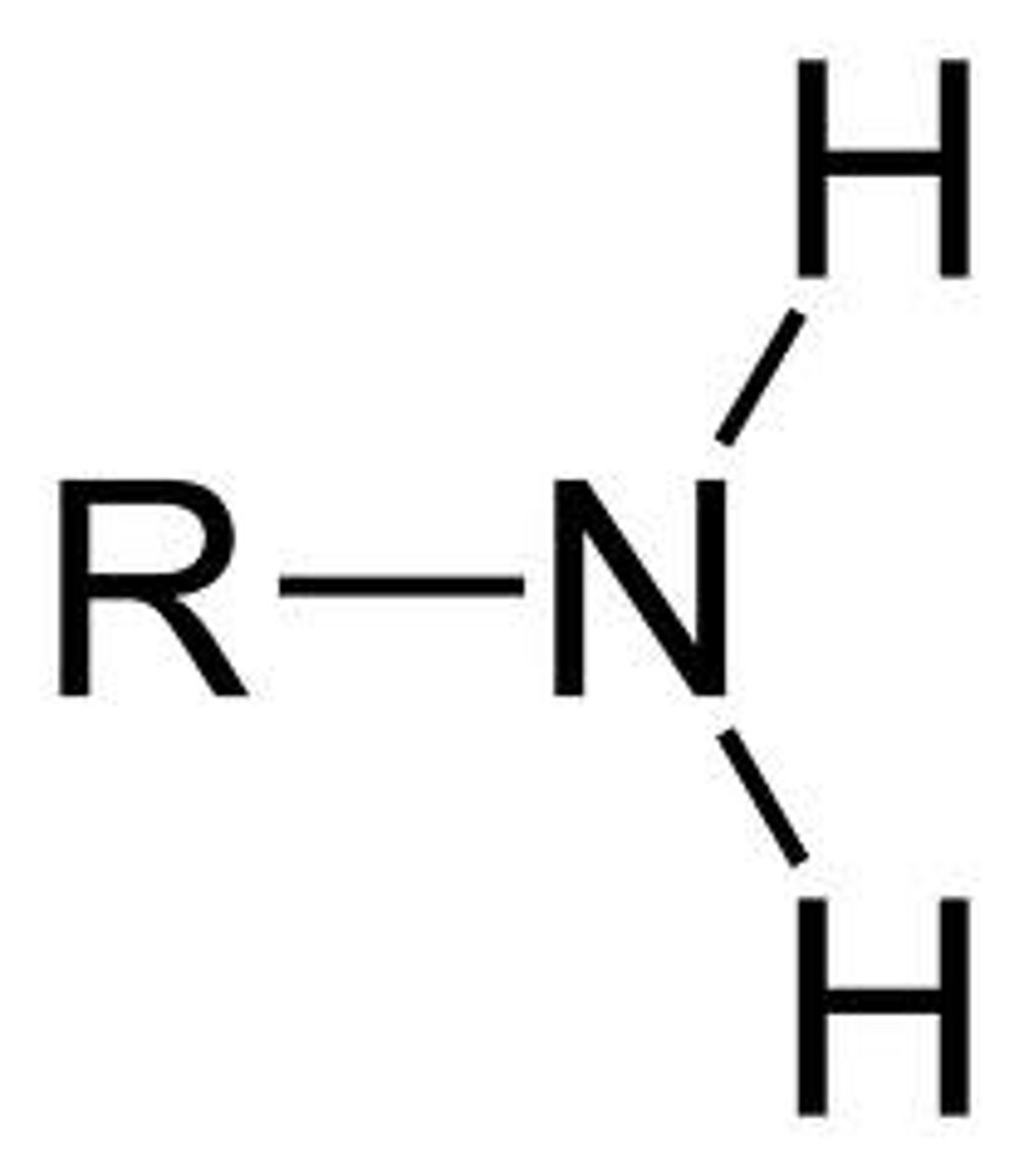
Take alkyl name of chain & ends in "-amine"
Begins with "amino-" (if paired w/ other functional group that alters ending)
Has a terminal N group
Amine
Amine
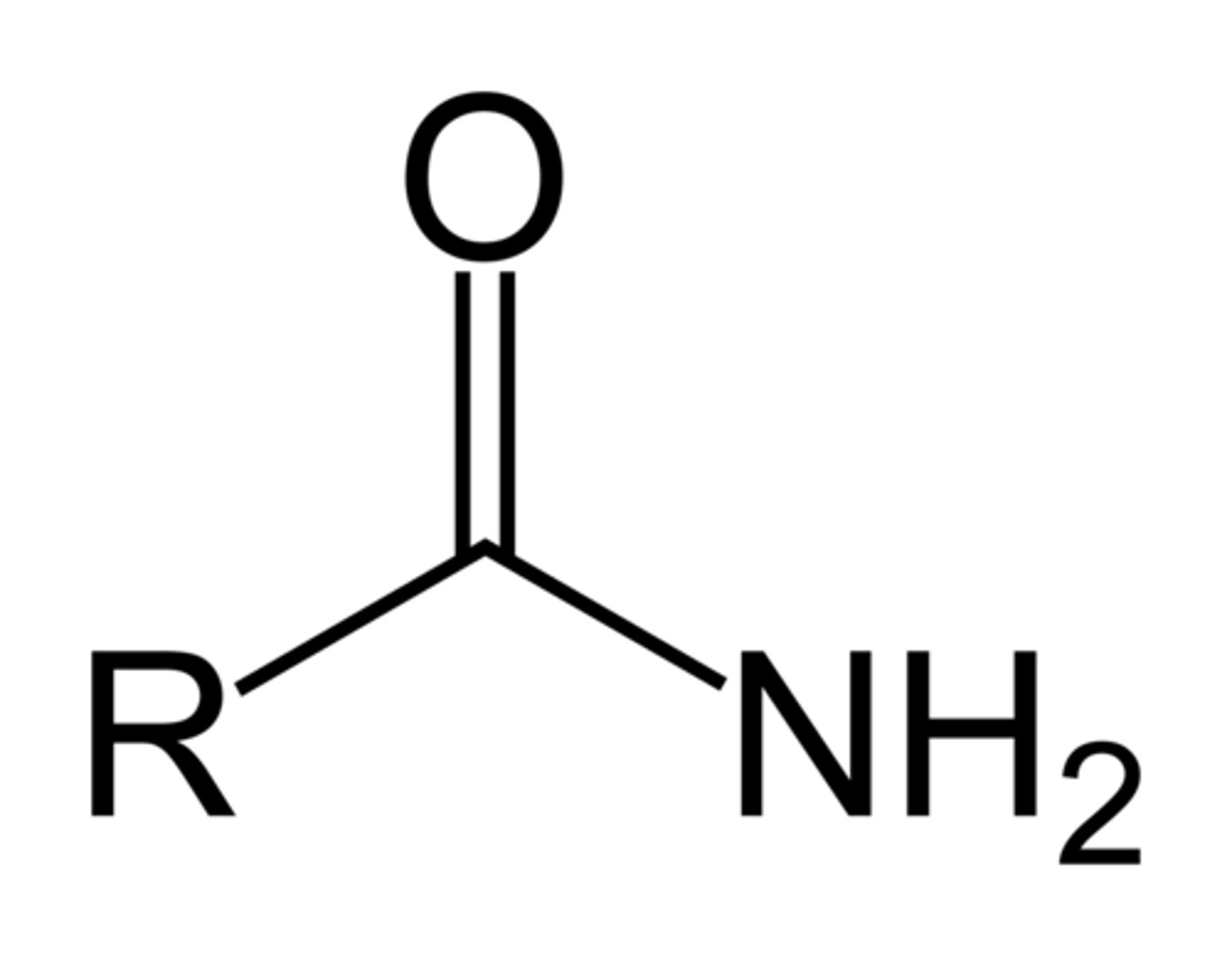
Ends in "-amide"
Has a terminal N group and carbonyl branch on the same carbon
Amide
Amide
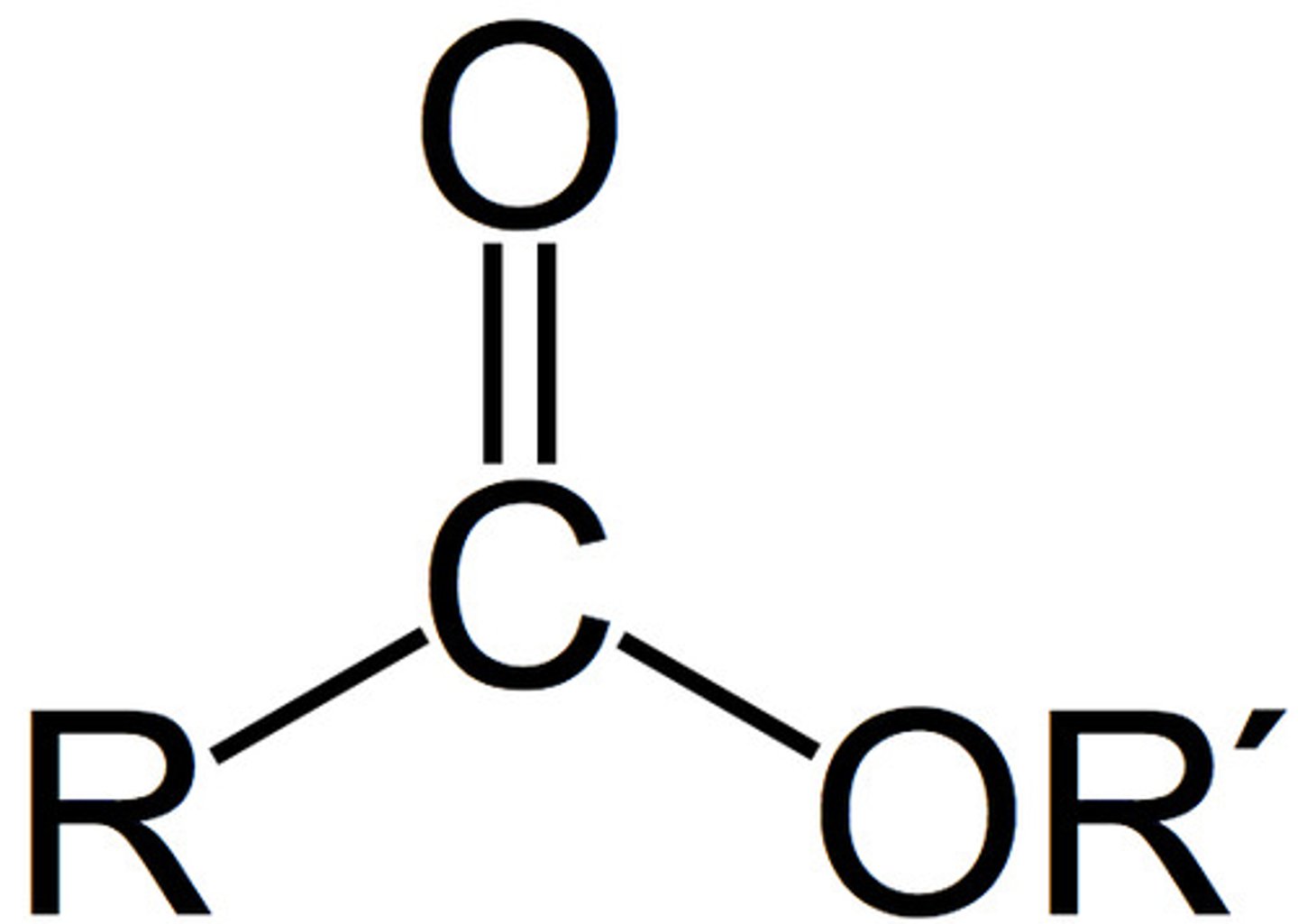
Formed by the combining of alcohol & carboxylic acid
Named by changing the alcohol name to ending "-yl" (replaces "an" as well) & changing acid name to ending "-oate" and dropping acid
Has an intermediate carbonyl & an O molecule interrupting the carbon chain
Ester
Ester
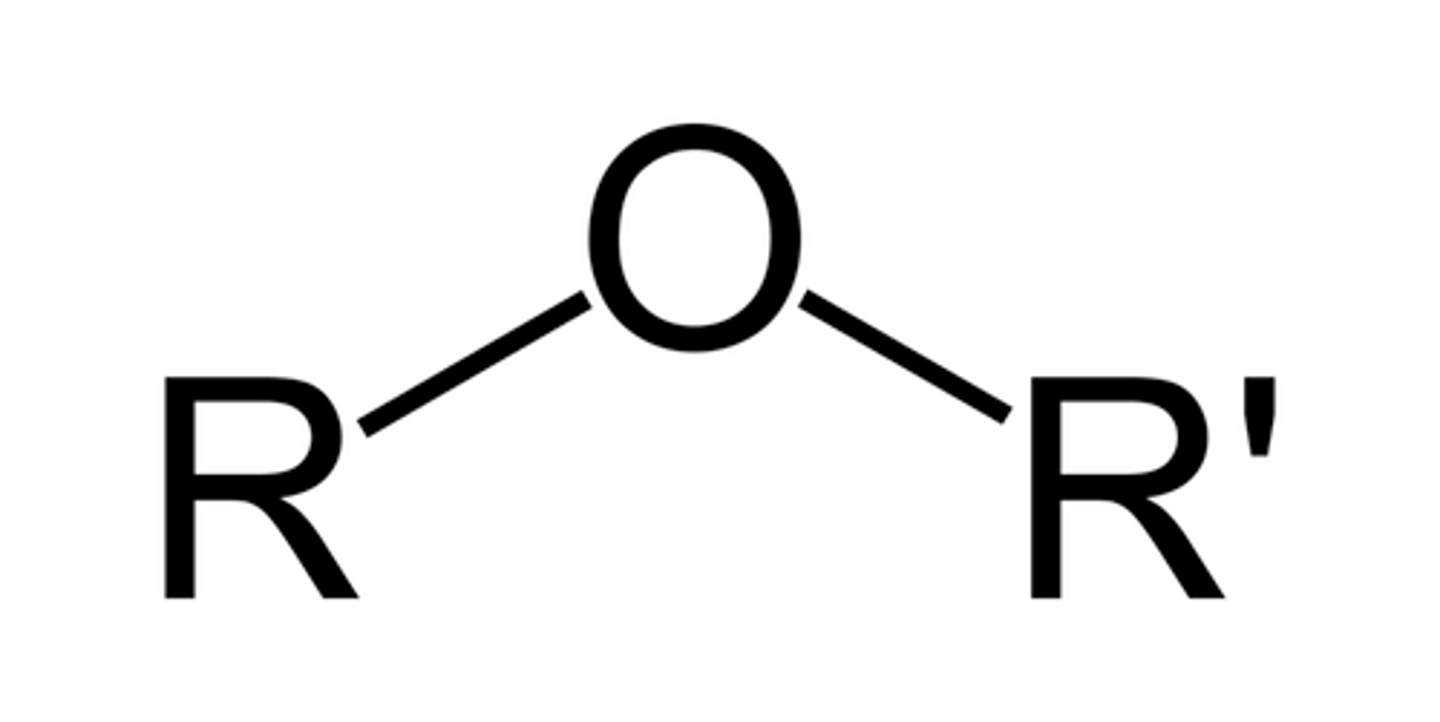
Broken into two (either side of the O)
- Shorter chain ends in "-oxy"
- Longer retains the name of the chain
Ether
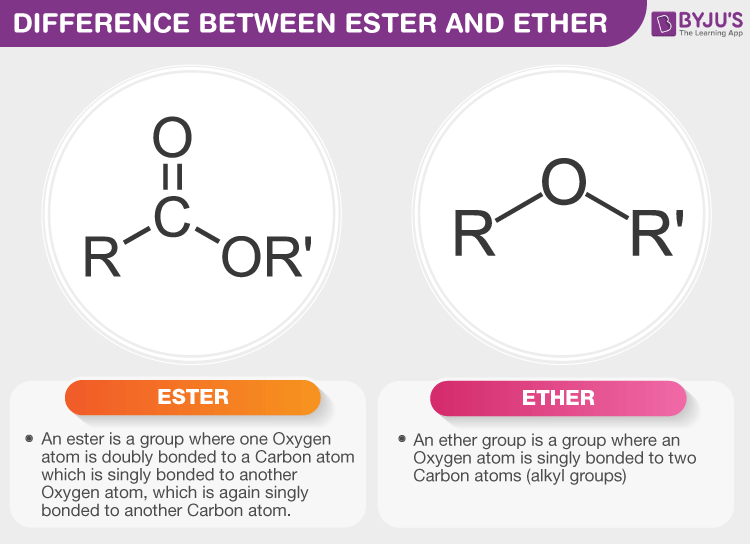

Ether
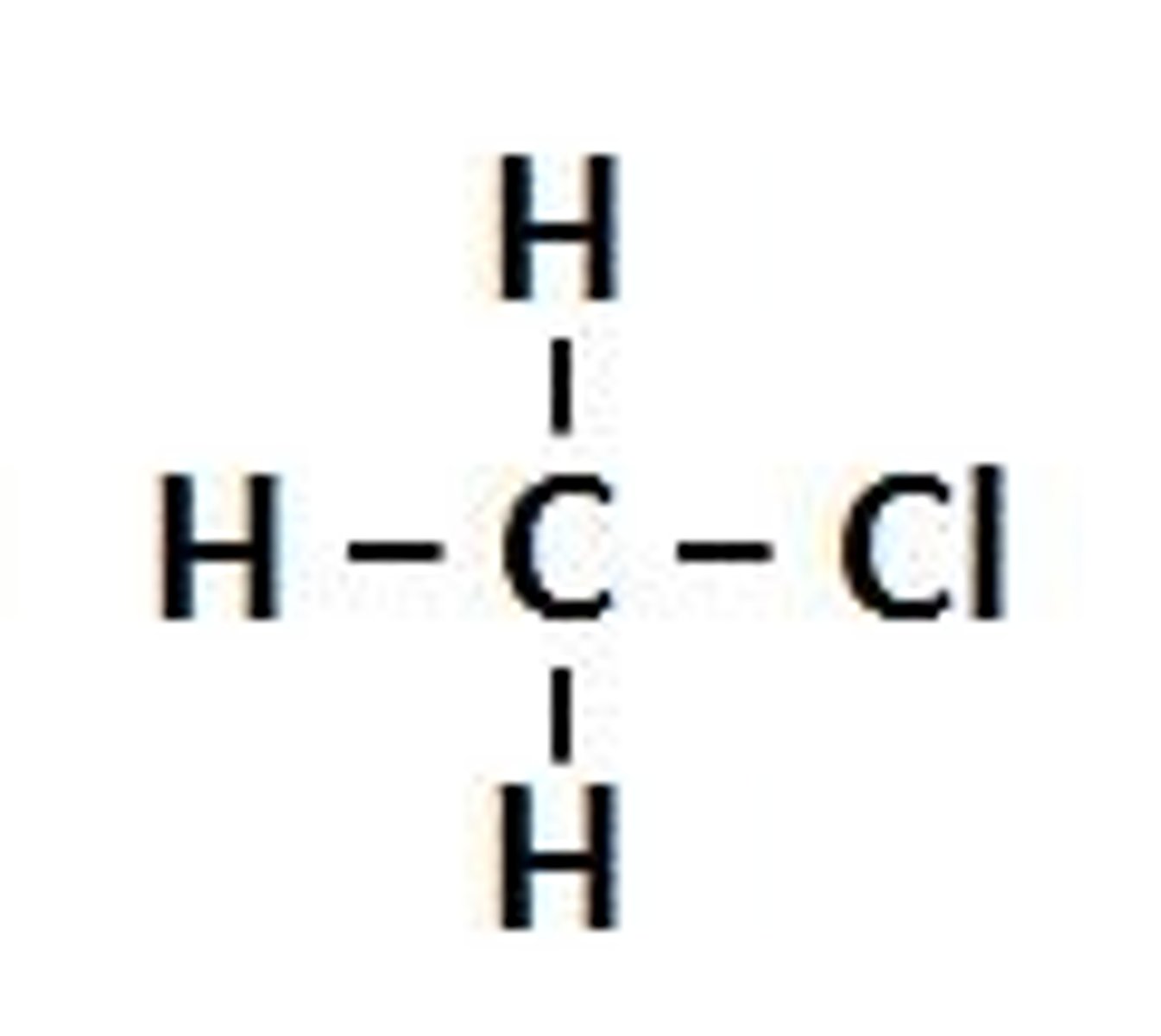
Name= (alkane name + chain name)
Halogenoalkane
Halogenoalkane (chloromethane)
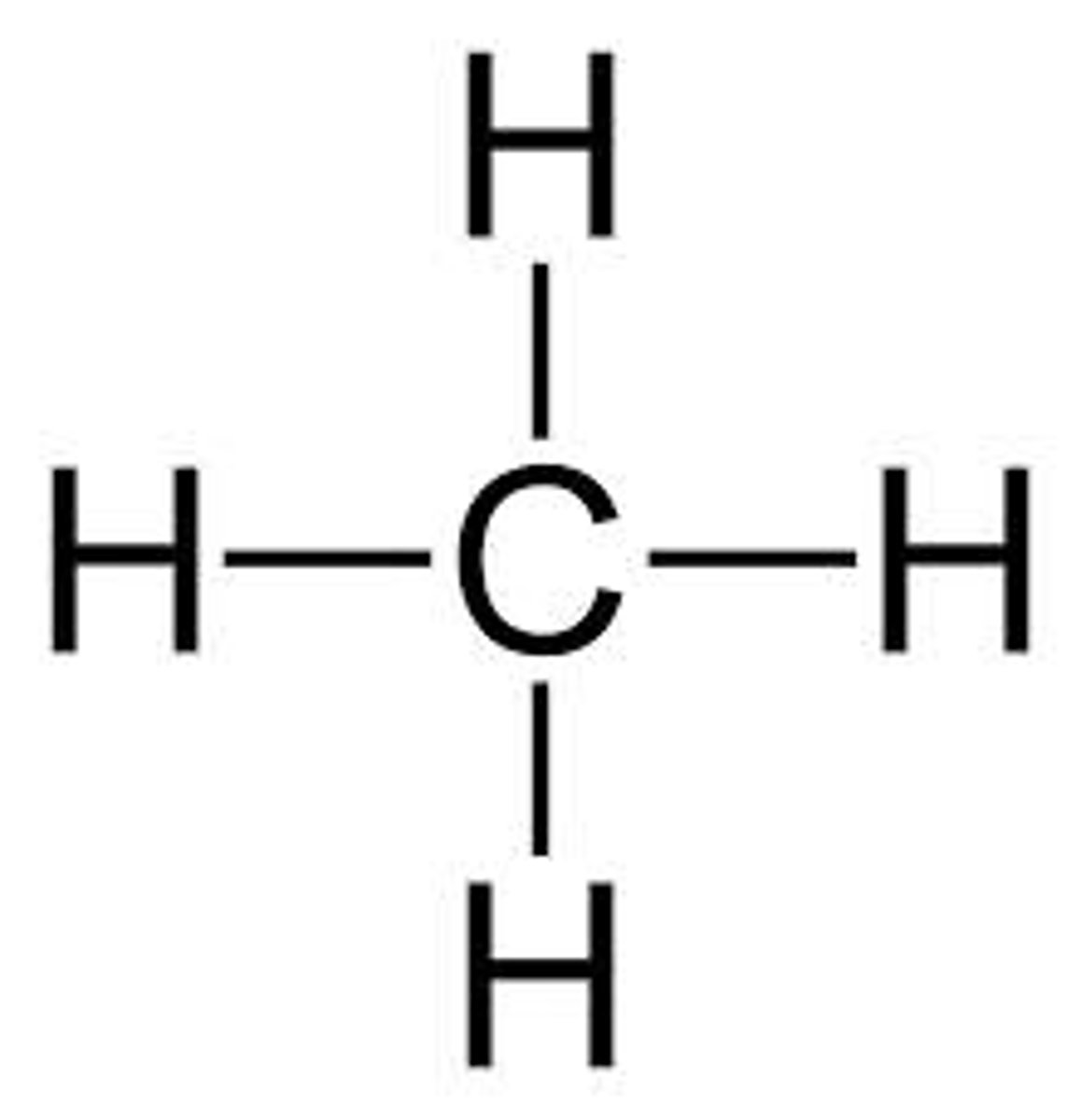
methane
1-carbon chain
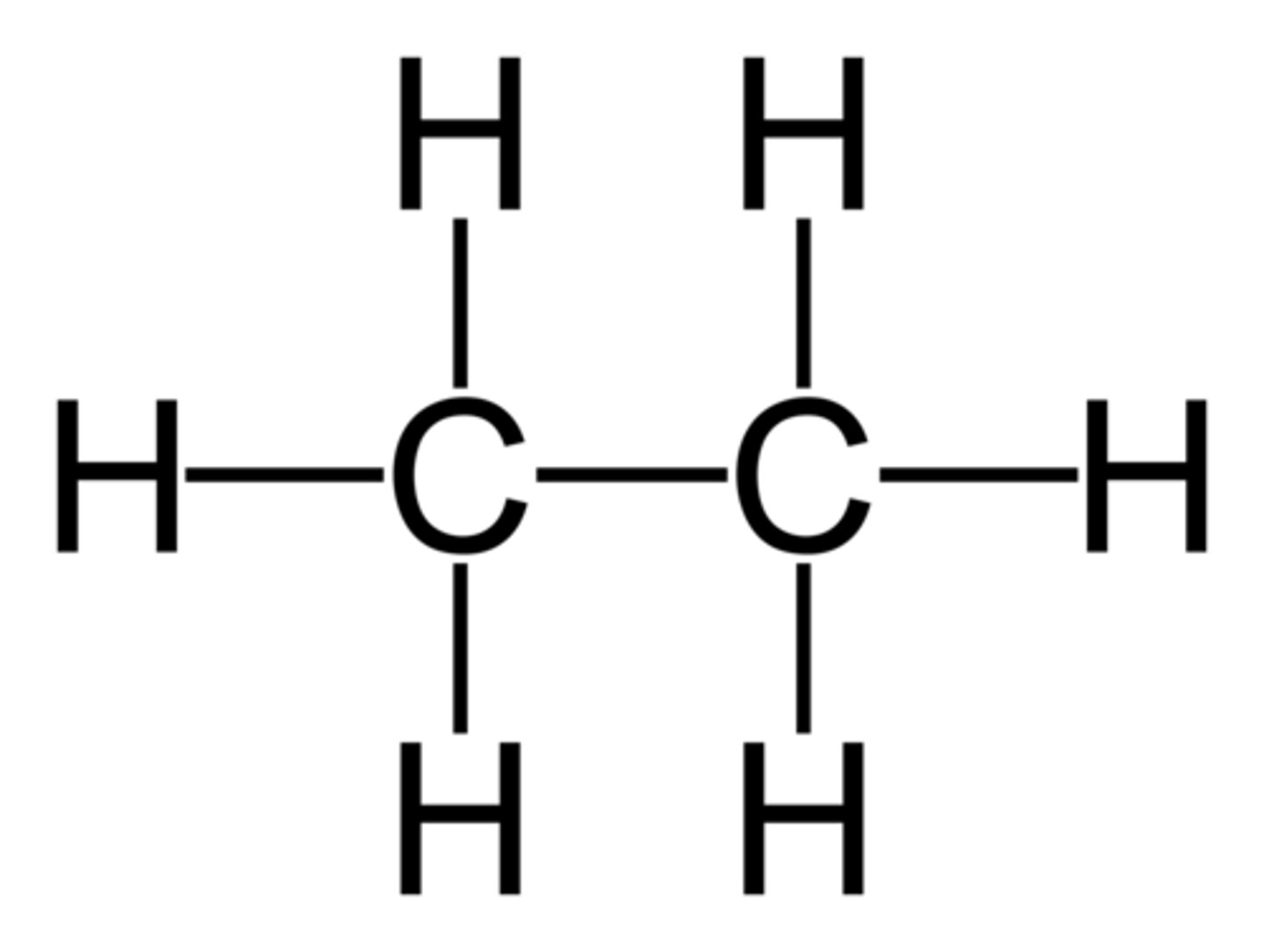
ethane
2-carbon chain
propane
3-carbon chain
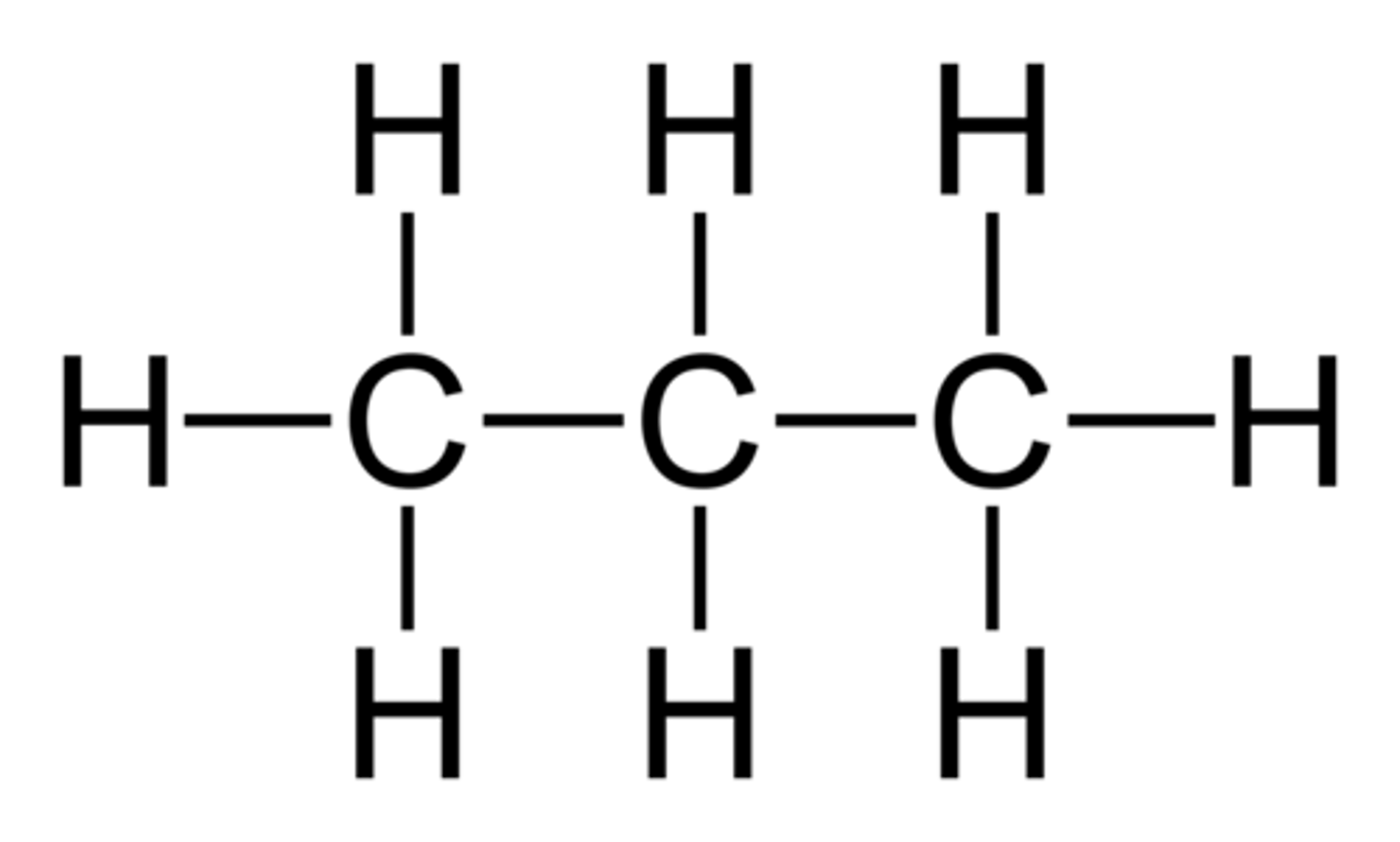
butane
4-carbon chain
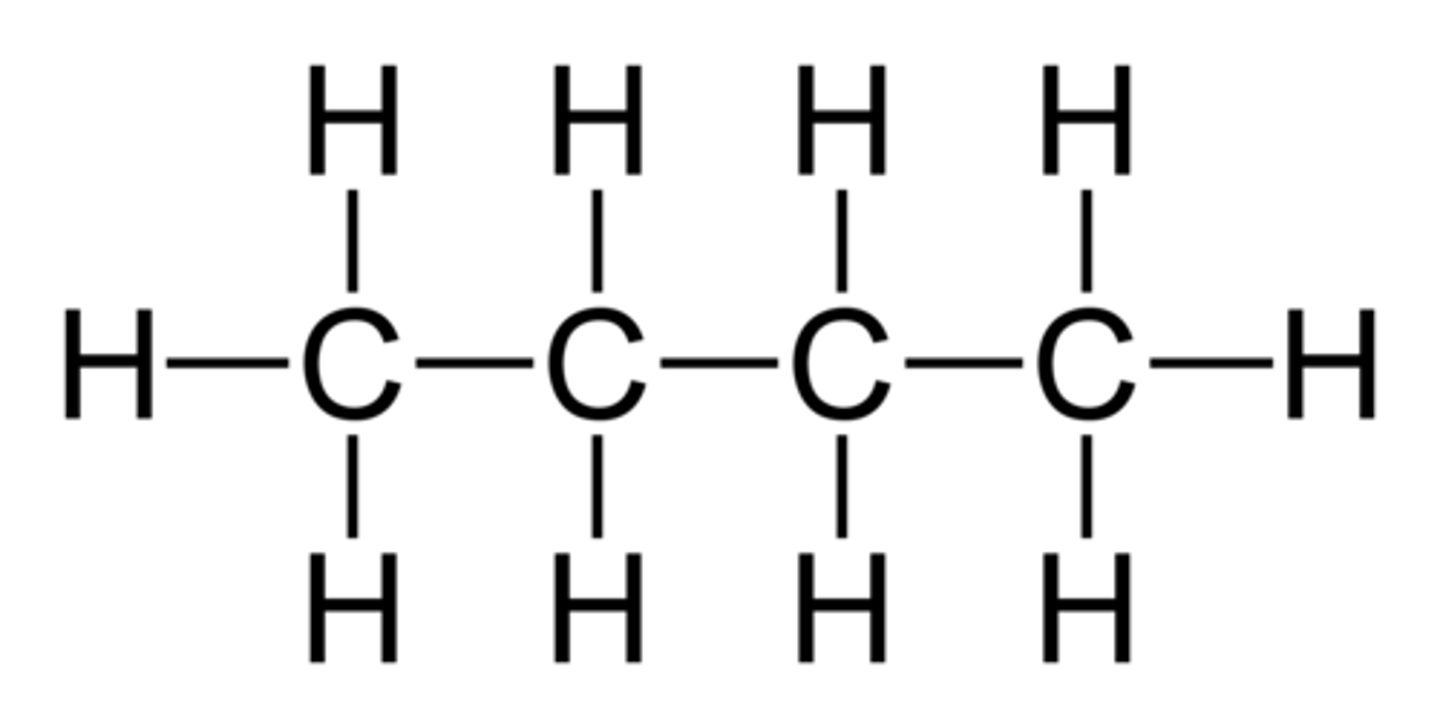
pentane
5-carbon chain
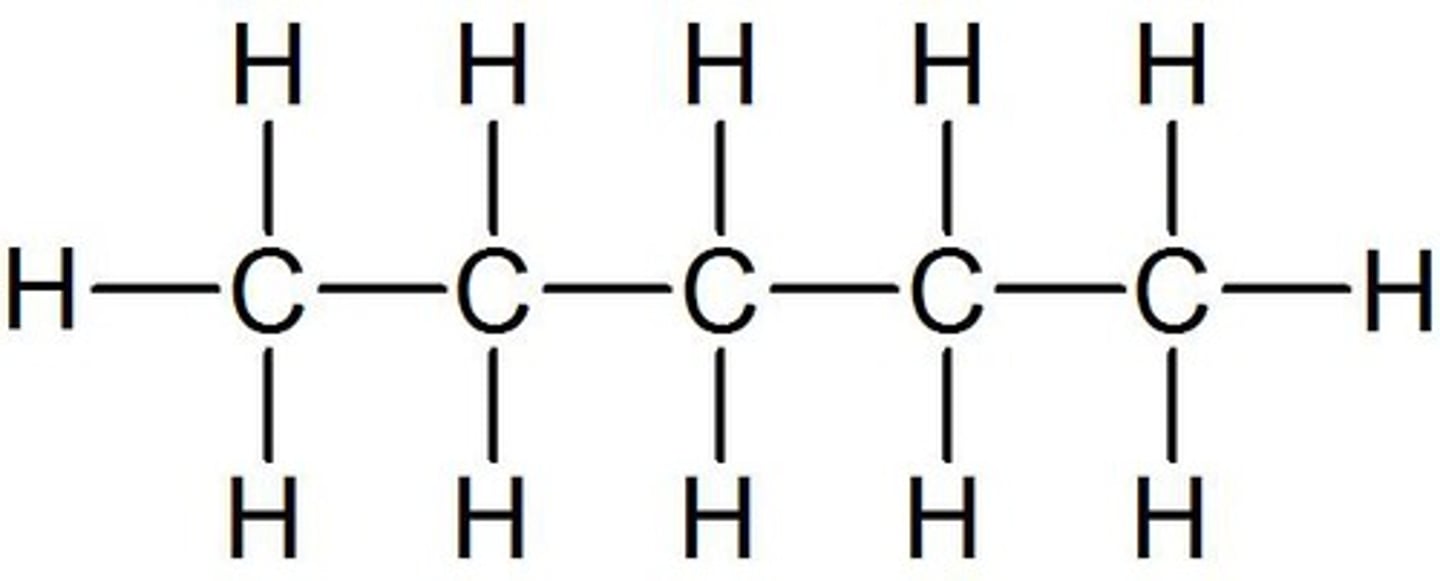
Hexane
6-carbon chain
-ane
Carbon chain that contains only single bonds
-ene
Carbon chain that contains at least one double bond
-yne
Carbon chain that contains at least one triple bond
Drop "-ane" and replace with "-yl"
Alkyl names
cyclo-
The prefix if a carbon ring
1. Count longest carbon chain and name accordingly (including ane, ene, or yne for bond lengths)
2. Number functional groups (f.g.) to give the lowest set of numbers (the # of the C that they brach off of)
3. If more than one f.g., use a number prefix
4. Arrange in alphabetical order ignoring number prefixes, and always put chain name at the end.
5. If a hydrocarbon is in the functional group, then name ends in -yl (alkyl group)
6. Dashes between numbers and letters, commas between numbers.
IUPAC Naming steps
1. Aldehydes, Carboxylic Acid, and Amides (terminal endings)
2. Keytones
3. Double bond
4. Alkyls, halogenoalkanes, alcohols
Naming Hierarchy