Chemistry - Structure and Bonding shapes
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
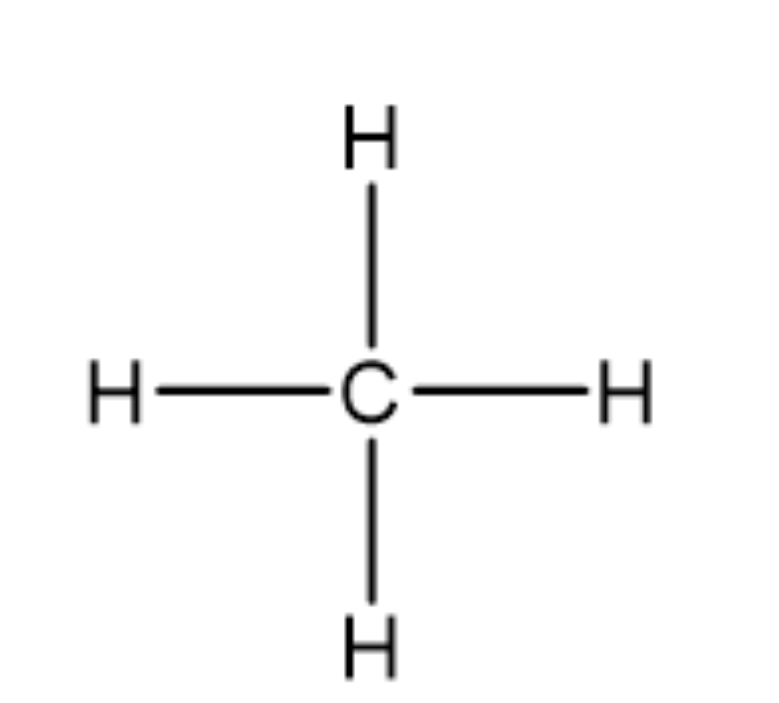
TETRAHEDRAL:
4 regions of negative charge around the central atom
all regions bonded
bond angles = 109 degrees
2
New cards
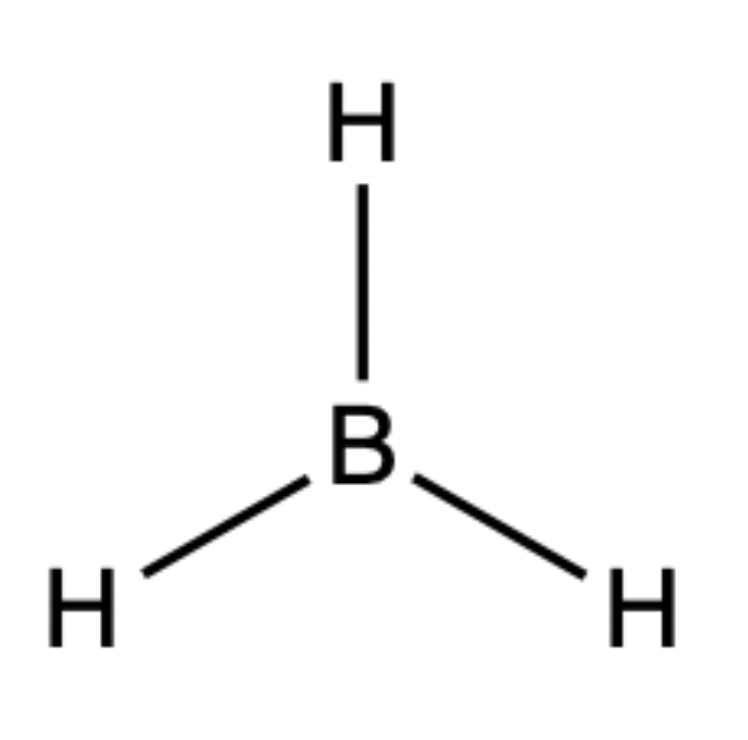
TRIGONAL PLANAR:
3 regions of negative charge around the central atom
all regions bonded
bond angle = 120 degrees
3
New cards
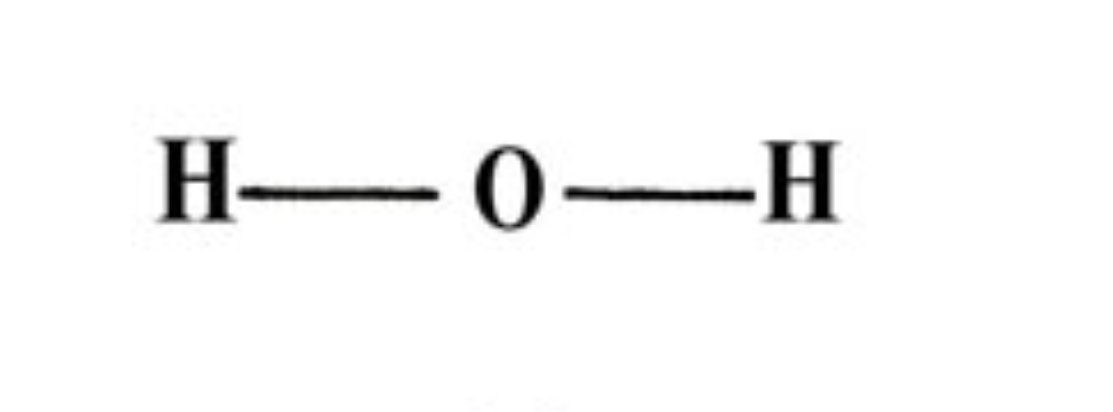
LINEAR:
two regions of negative charge around the central atom
all regions bonded
bond angles = 180 degrees
4
New cards
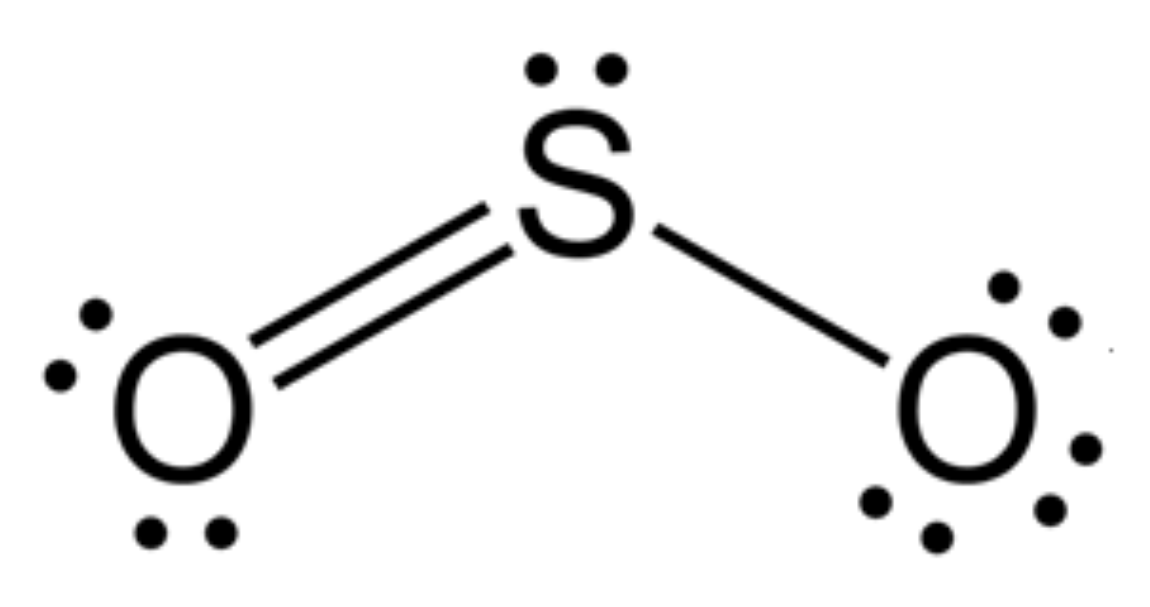
BENT (3 REGIONS):
three regions of negative charge around the central atom
two bonded, one unbonded
bond angle = 120 degrees
5
New cards
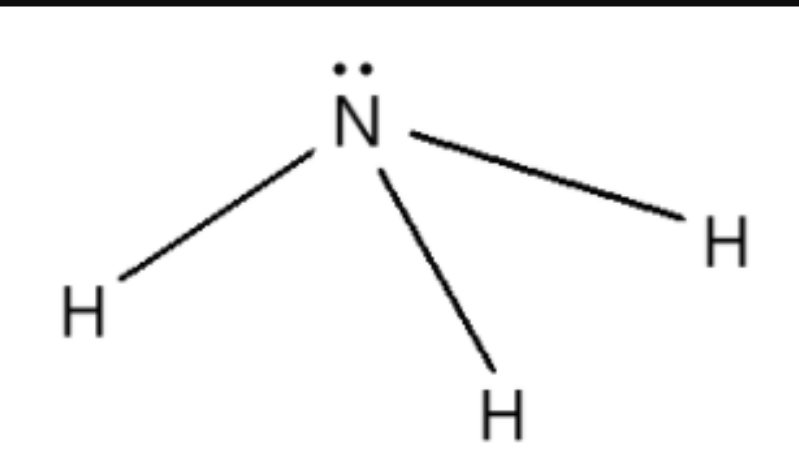
TRIGONAL PYRAMIDAL:
4 regions of negative charge around the central atom
three bonded, one unbonded
bond angle = 109 degrees
6
New cards
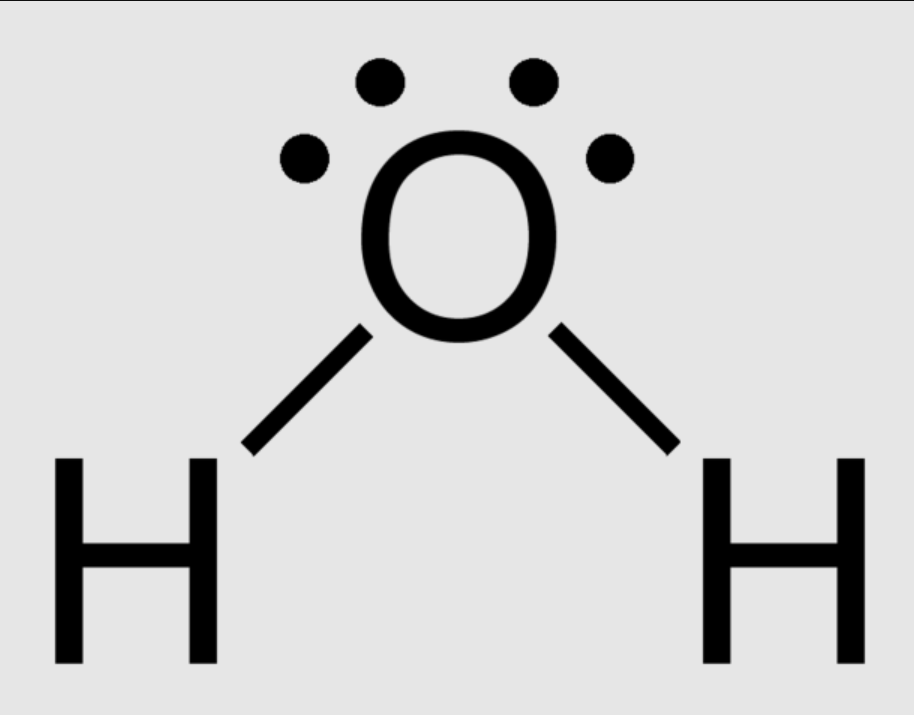
BENT (4 REGIONS):
4 regions of negative charge around the central atom
two bonded, one unbonded
bond angle = 109 degrees