solids, liquids, gases
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
solids
particles are closely packed in a lattice (fixed arrangement)
fixed volume
vibrates
cannot be compressed
liquids
particles are close together in a random arrangement
particles slide past each other
cannot be compressed
takes the shape of the container its in
gases
particles are spread far apart (arrange randomly)
can be compressed
moves really quickly
when temperature increases
or when pressure decreases
volume of a gas increases
when pressure increases
volume of a gas decreases
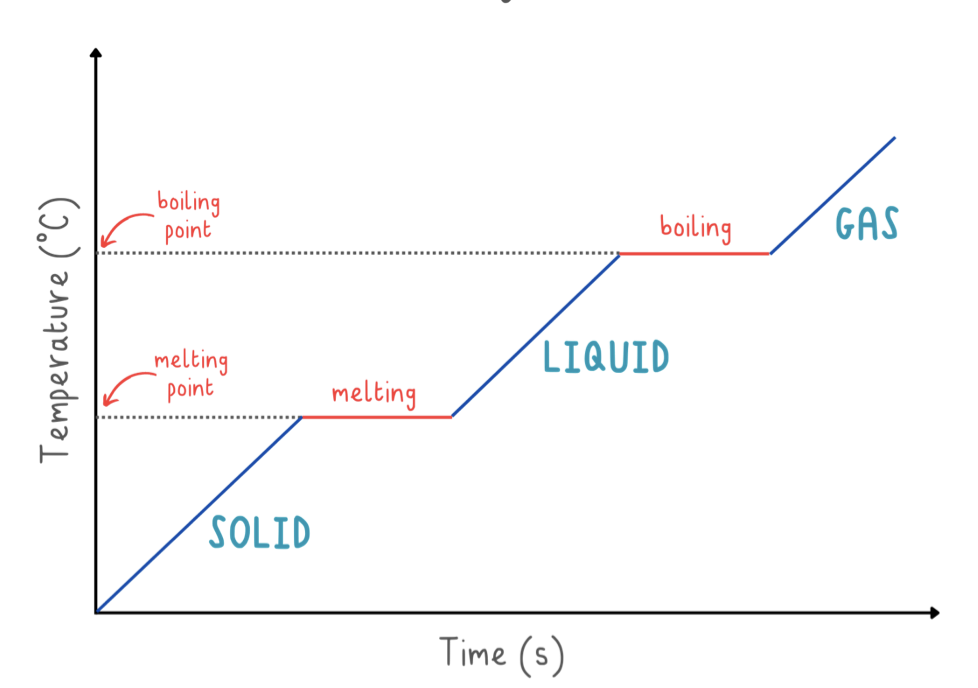
heating curve
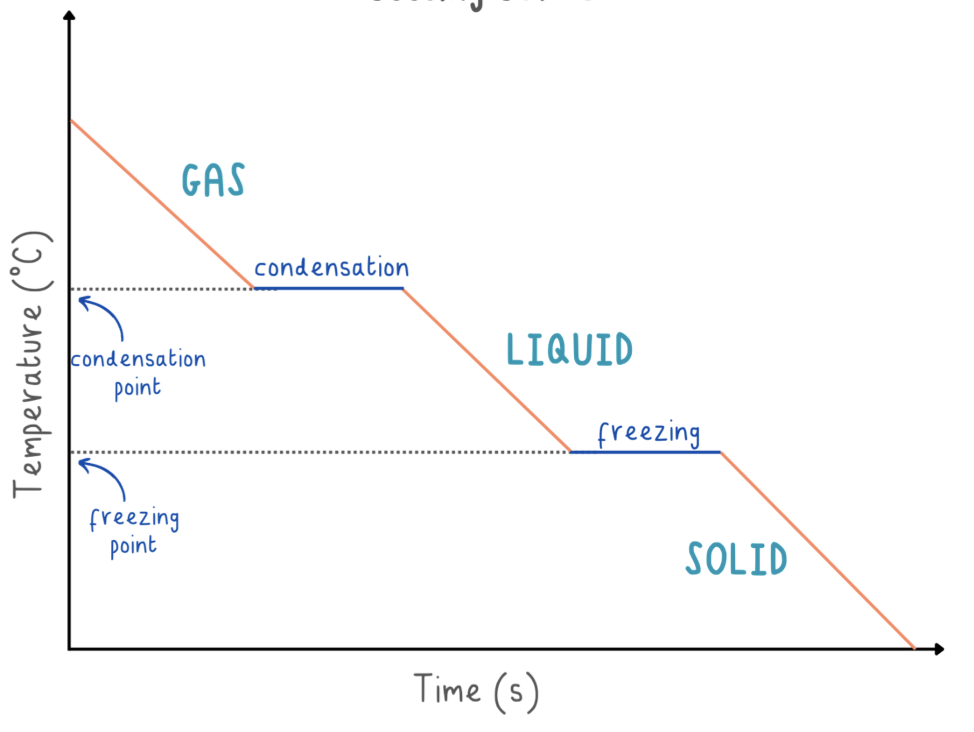
cooling curve
liquid state
between melting and boiling point
solid state
below its melting point
gas state
above its boiling point
sublimation
solid to gas
difference between boiling and evaporating
boiling happens over a specific temperature while evaporation happens over a range of temperatures
kinetic particle theory
all matter is made of small particles that are in random motion
if the temperature is lower than the melting and boiling point
it is a solid
if the temperature is between the melting and boiling point
it is a liquid
if the temperature is higher than the melting and boiling point
it is a gas
arrangement of particles for liquid
irregular arrangement
arrangement of particles for gas
spread far apart (no arrangement)
arrangement of particles for solids
in a lattice