CHEM111: Module 10 Acid-Base Equilibrium
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
how can you measure the strength of acids & bases (other than pH)
use the pKa/pKb scale
this = -log10(Ka), providing values on a 1-10 (Strong-weak) scale
Ka/Kb is the equilibrium constant, so higher Ka=more products=more dissociation=stronger acid/base (so -log of it gives a pKa scale of strong-weak)
how does the equilibrium constant (Ka/Kb) take form for acids/bases
why is this relevant
what can we use this to find
K = [products] / [reactants]
Ka = [H+] [A-] / [HA]
Kb = [OH-] [B+] / [BOH]
this is relevant as high Ka=lots of products=lots of dissociation=strong acid/base
we can use this to find pKa (-log) & pH (rearrange)
how do you calculate pH from Ka (For both weak & strong acids)
pH is -log[H+], so we want to use this to find [H+]
Ka = [H+] [A-] / [HA]
Weak acids =
we know [A-]=[H+] (donating 1H+ forms 1A-), so Ka becomes = [H+]² / [HA]
we can assume [HA]equil=[HA]time 0
then rearrange and [H+] = sqroot Ka x [HA]
Strong acids =
complete dissociation of HA into [H+] & [A-]
so [H+]=[HA]
then just take the -log, and pH is provided
who came up with pH and when?
what is pH
Soren Sorensen (1800s) said that [H3O+] = 10^pH
he said a pH=7 is neutral, while <7=acidic, and >7=basic
therefore pH is the -log of [H3O]+, providing a way to measure acidity / basic of a solution
what did Arrhenius suggest about acids / bases
and when?
1884
he suggested acids deprotonate to produce H+ in aqueous solutions
and bases protonate, to leave behind OH- in solutions
and these can be complete or incomplete (equilibrium) dissociates (weak/strong bases / acids)
what did Bronsted & Lowry suggest about acids / bases
and when?
1923
they went a step further from Arrhenius and suggested acids are proton donors, while bases are proton acceptors
thus broadening the definition, suggesting acid/base reactions involve transferring a proton (acid→base transfer)
what did Lewis suggest about acids / bases
and when?
1923
he suggested acids accept electron pairs, and that bases donate electron pairs (opposite to proton transfer) - producing a base adduct (species formed from this)
this extended the definition to look at electron movement instead of proton transfer
acids / bases described this way are ‘lewis acid’ and ‘lewis base’
whos definition of acids / bases is most commonly used today
the Bronstead & Lowry definition, where acids are proton donors while bases are proton acceptors, and acid/base reactions involve proton transfer from acid→base
what are acids
how do they behave in aqueous (w/ Water) solutions)
acids are species that deprotonate, they dissociate to produce H+
this is usually from a breaking of an -O-H bond (except for H halides, where this H-halide bond is broken) - leaving behind an -O- on the molecule
this H+ is attracted by water’s negative dipole (on O), forming H3O+ (hydronium ion)
what is the difference between strong and weak acids
give examples of each type
strong acids completely dissociate / are completely ionised, into the acid ion and H+, leaving behind no original acid
e.g. HCl, HBr, HI, HNO3, HClO4, H2SO4
weak acids only partially dissociate / are ionised into the acid ion and H+, so some original acid remains, and an equilibrium mixture of species is formed
e.g. HF, most organic acids, H3PO4
what is an example of a molecule that behaves like an acid, but is not truly an acid
metal ions in aqueous solutions are surrounded by hydration shells (associations with water molecules)
these can produce acidic solutions, as additional water molecules may interact with the hydration shells to form stronger H interactions
therefore accepting an H+ and the solution becomes more acidic (more H3O+ produced)
what are bases
give examples of weak and strong bases
bases are species that protonate, they dissociate to produce an ion and OH-, as they accept an H+ from water
strong bases include: NaOH, KOH, LiOH, Ca(OH)2, Ba(OH)2, Sr(oh)2
weak bases include: NH3, amines (R-NH2), CO3²-)
what are conjugate acid/base pairs
these are the species formed when an acid or base dissociates, which can then go on to act as the opposite (acid → conjugate base, base → conjugate acid)
this is due to acids deprotonating, so they can then protonate, and bases protonate, so they can then deprotonate
e.g. H2O (base) protonates to form H3O+, which can then act as an acid and deprotonate
strong acids / bases form weak conjugates and vice versa, due to Ka increasing as Kb decreases and vice versa
what is an amphiprotic species
a species that can either donate or accept a proton
e.g. H2O, can donate a proton and become OH-, or accept and become H3O+
how does the example of corals dissolving due to climate change, relate to acids?
more CO2 in atmos → more dissolves into carbonic acid (Weak) in the sea → this dissociates to form hydrogen carbonate + H+
this hydrogen carbonate reacts with CaCO3 structures of coral → CaCO3 dissolves as it protonates to form aqueous calcium hydrogen carbonate
this also forms CO2 as a byproduct, which then adds to the increasing CO2 and the cycle continues
what is the Henderson Hasselbalch equation?
what can we use this to find?
pH = pKa + log ([A-] / [HA])
we can use this to find pH, pKa, etc
depending on the information provided to us
what is a polyprotic acid
provide an example
these can deprotonate multiple times, donate multiple protons
e.g. H2SO4 → HSO4- → SO4²-
what is a buffer solution
what is it made of
what does it do
a solution that contains a weak acid, and its conjugate base
it is resistant to pH change when a strong acid / base is added, therefore acting as a pH buffer
how do buffer solutions work?
these are solutions of a weak acid and its conjugate base, in equilibrium
adding a strong acid (adding H+) will shift equilibrium to the left, to use up H+ and counteract the addition, therefore resisting a pH change as H+ still doesn’t increase
adding a strong base (adding OH- and decreasing H+ as it reacts to form H2O) will shift equilibrium to the right, to make more H+ and counteract the removal, thus resisting a pH change as H+ still doesnt decrease
eventually the added strong acid / base will change pH (buffer action stops) when equilibrium shifts too far (Weak acid conc. decreases too much)
how do you find the pH of a buffer solution
how do we determine a buffer to maintain a certain pH
the pH will be close to the pKa of the weak acid
so use the Ka for the buffer system to calculate the pKa, and this will resist the pH change of pH close to this pKa
to find the exact pH, use the Henderson Hasselbalch Equation (pH=pKa+log( [A-]/[HA] )
what happens if you dilute a buffer
diluting a buffer does not change the ratio of [H+][A-] : [HA] (used to determine the Ka) so Ka won’t change, therefore pH won’t change
eg water entering cells (body’s buffer)
how are buffer systems involved with red blood cells in the body
red blood cells contain haemoglobin, which pick up O2 at the lungs, transfer it to tissue, then remove the CO2 from the tissue
the bicarbonate buffer regulates this transfer, as CO2 dissolves in the cell’s cytoplasm → forms carbonic acid → releases H+ → associated with haemoglobin & 2O → cause shape change and O2 release
at the lungs, the reverse occurs, depleted haemoglobin associates with O2 → CO2 pops off the carbonic acid → CO2 release to be breathed out the lungs
therefore CO2 removal enabling O2 release
what is an indicator
describe an example in the natural world
solutions that change colour due to changes in pH, each with different pH ranges (ranges where they change colour)
e.g. hydrangeas change colour based on the pH of the soil they are in, acidity (and Al present in soil) will turn them blue
how does phenolphthalein indicator work
this is a weak acid, which is diprotic (can release 2H+ to form a dibasic ion), so in equilibrium between the two
is colourless when protonated, is bright magenta when deprotonated (when dibasic)
adding a base (OH-) will remove H+ from the acid to form water, causing equilibrium to shift to the right (form more H+ & base) so magenta indicates basic
adding an acid (H+) will favor the reverse to use up the H+ (form more acid) so colourless indicates acidity
8.3-10 pH range
how does 2-methyl Orange indicator work?
weak acid in equilibrium with its conjugate base
red in acidic form, orange in basic form
when acid is added (H+) reverse is favored, conjugate base is protonated, and solution turns red
when base is added (OH- reacts to remove H+ and form H2O) the forwards is favored, more conjugate base formed, so turns orange
pH range 3.1-4.4
describe the carbonic acid / hydrogen carbonate buffer system
CO2 + H2O <==> H2CO3 <==> H+ + HCO3-
so adding CO2 to water forms H2CO3 in equilibrium with H+ and HCO3- (its conjugate base)
adding an acid (add H+) will shift to the left, using up H+ to resist pH change
adding a base (adding OH- to remove H+) will shift to the right to form more H+ and resist pH change
what is another name for titrations in the lab
name the equipment used, and each of their purposes
Volumetric analysis
equipment used includes
burette (various volumes and classes for accuracy, filled with base which it allows to be gradually added into an acid beaker)
volumetric flask (to make up a solution to a certain volume)
pipette (glass, measures specific amounts of chemicals)
beaker
indicator solution (indicates near end point)
pH meter (glass electrode in the beaker being titrated on to measure pH)
what does a titration curve display
how do these differ between titrations of (strong acid/strong base) and (weak acid/strong base)
plotted pH values of the titration, against the volume of base added, including indication of the equivalence and end point
strong acid / strong base:
low pH at the start due to strong acid, which rises slowly
until reaching equivalence point (Steepest part of the graph) at pH7, where it steeply rises, as it neutralises
then afterwards it flattens off at a high pH (strong base)
weak acid / strong base:
higher pH at the start due to weak acid, which rises slowly as it resists pH change (buffer region)
this contains half equivalence point (Veq/2) where pH=pKa ([HA]=[A-])
it then rises steeply, reaching equivalence point (steepest part), at a pH higher than 7
it then flattens off at high pH just like strong acid curve (due to strong base)
in an acid/base titration, what is the difference between an end point and an equivalence point?
how do we get these as close as possible, and why is that important?
an end point refers to when the indicator changes colour, to signify a certain pH has been reached within the solution
an equivalence point refers to when number moles acid = number of moles base (or according to reaction stoichometry), where neutralisation occurs of OH-&H+ to form H2O
it is important these are close as possible, so we can figure out the pH of the equivalence point (differs from pH7 for weak acids/strong bases), and use this to figure out unknown concentrations
this is done by choosing an indicator with a pH colour change range, over the equivalence point, by ensuring its pKa is close (the point where indicator is in middle of colour change, [HA]=[A-]=[H+]=Ka, so pKa=pH)
how do equivalence points differ between
strong acid / strong base titrations
weak acid / strong base titrations
strong acid / strong pace
equivalence point will be pH of 7
this is because both species fully dissociate into OH- and H+, therefore at this point there is an equal balance of [OH-] and [H+], which will react and neutralise to form water
this results in a neutral pH of 7
what is a half equivalence point
what can we use this to find
only in weak acid/strong base titrations, the part of the curve where V = Veq/2
this is also where pH=pKA ([HA]=[A-]), so we can use this to find these
why is the equivalence point at a higher pH for weak acid x strong base titrations (compared to strong acid x strong base Eqiv=pH7)
the weak acid dissociates into a strong conjugate base, which forms OH- and makes the pH more basic (higher at equivalence point)
base is stronger, so it ‘wins’ and pulls equiv pH to a more basic value
how to find pOH
how does this differ from pH
pOH is -log[OH-], so the opposite of pH
so to find, pOH = 14-pH
what is the expression for Kb
[OH-] [HB+] / [B]
what is a common approximation we make for titration calculations, for concentrations of acids / bases at equilibrium?
for equilibriums where K is very small (equilibrium far to the left), we can assume that the [acid or base] at time 0, is the same as the [acid or base] at equilbrium
therefore we can use it to rearrage the equilibrium expression to find unknowns
how would you find concentrations of weak acids / bases after the titration has progressed some amount (not using the approximation that the conc is the same as time 0)
look at the amount of titrant added, and find the n for this (use V and conc), which will provide the n(H+) or n(OH-) (strong so it fully dissociates)
at equivalence point, this will equal the number of mols of conjugate formed (as pKa = pH, as [HA]=[H+])
then do n(weak at time 0) - n(conjugate), which will equal the n(weak) at the equivalence point
this can also be used to find pH or pOH
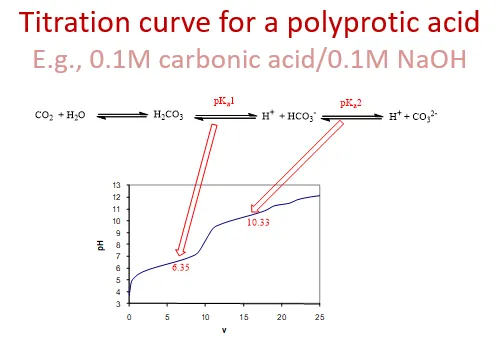
what would a titration curve look like for a polyprotic acid
will deprotonate multiple times, forming Ka1 and Ka2 (multiple equilibrium constants)
so graph will have multiple changes
e.g. carbonic acid
e.g. sulphuric acid
why does pH =pKa at half equivalence point?
because at half equivalence point, (HA) = (A-), so in the Ka expression they cancel out, and Ka simply becomes =(H+)
pKa is -log (Ka), and pH is negative log of (H+), therefore they equal