Reactions of Ethanoic acid with Sodium carbonate, Magnesium, Ethanol
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
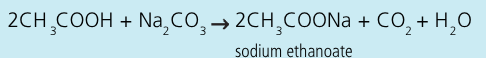
Key steps + observations in the procedure for the reaction of ethanoic acid with sodium carbonate:
place anydrous carbonate and ethanoic acid into a boiling tube connected to a solution of limewater
effervescence occurs, limewater goes milky showing the gas produced is carbon dioxide
a lit taper held at the mouth of the boiling tube is extinguished, carbon dioxide is present
Write an equation for the reaction between ethanoic acid and sodium carbonate
Write an equation for the reaction between carbon dioxide and limewater:
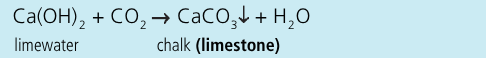
Key steps + observations in the procedure for the reaction of ethanoic acid with magnesium:
place magnesium metal into a solution of ethanoic acid
effevervescence occurs and a lit taper is held above the mouth of the test-tube
a squeakly pop, means that hydrogen gas is produced
Write an equation for the reaction between ethanoic acid and magnesium:
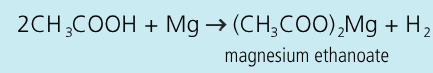
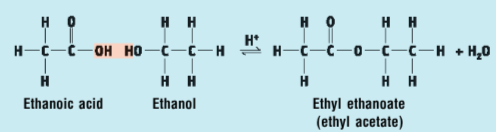
Key steps + observations in the procedure for the reaction of ethanoic acid with ethanol:
add ethanol and ethanoic acid together with some sulfuric acid (catalyst) in a test tube
observe that there is no colour change and a fruity smell is produced to the the ester (ethyl ethanoate) formed
Draw the structures in the reaction between ethanoic acid and ethanol + indicate the oxygen and hydrogens that form a water molecule