reaction 2 metal oxide with an acid
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
metal oxide + acid →
salt and water
Neutralisation reaction
Method
Using pH paper determine the pH of the acid at the start, record this in your results.
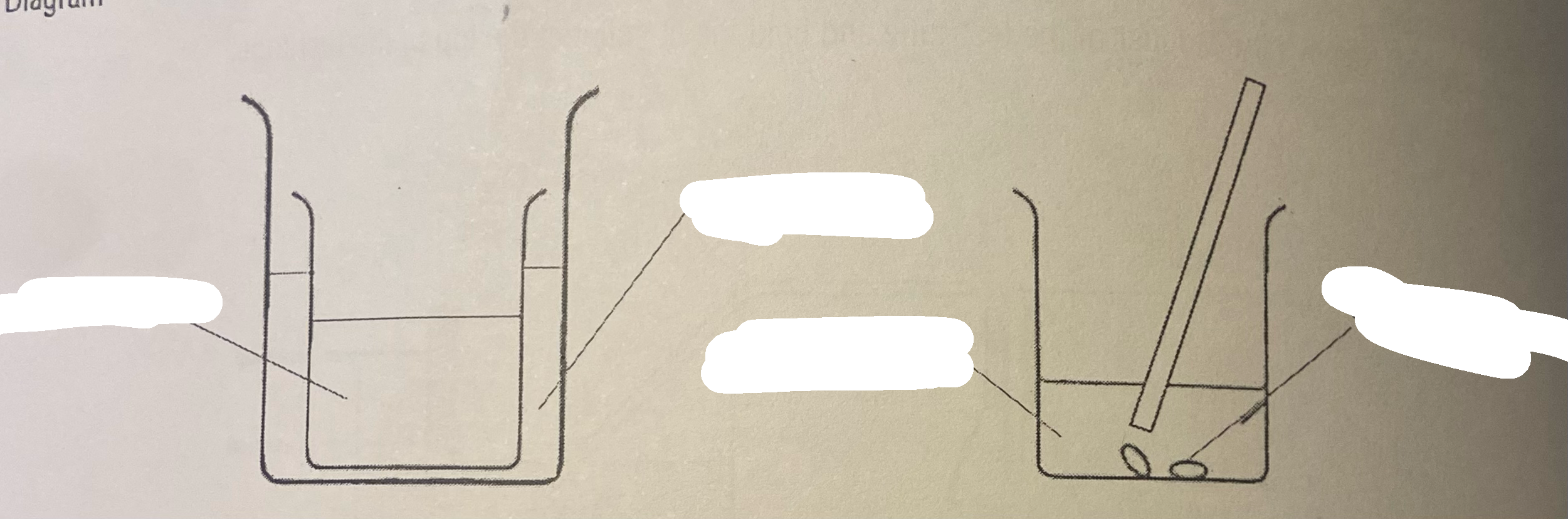
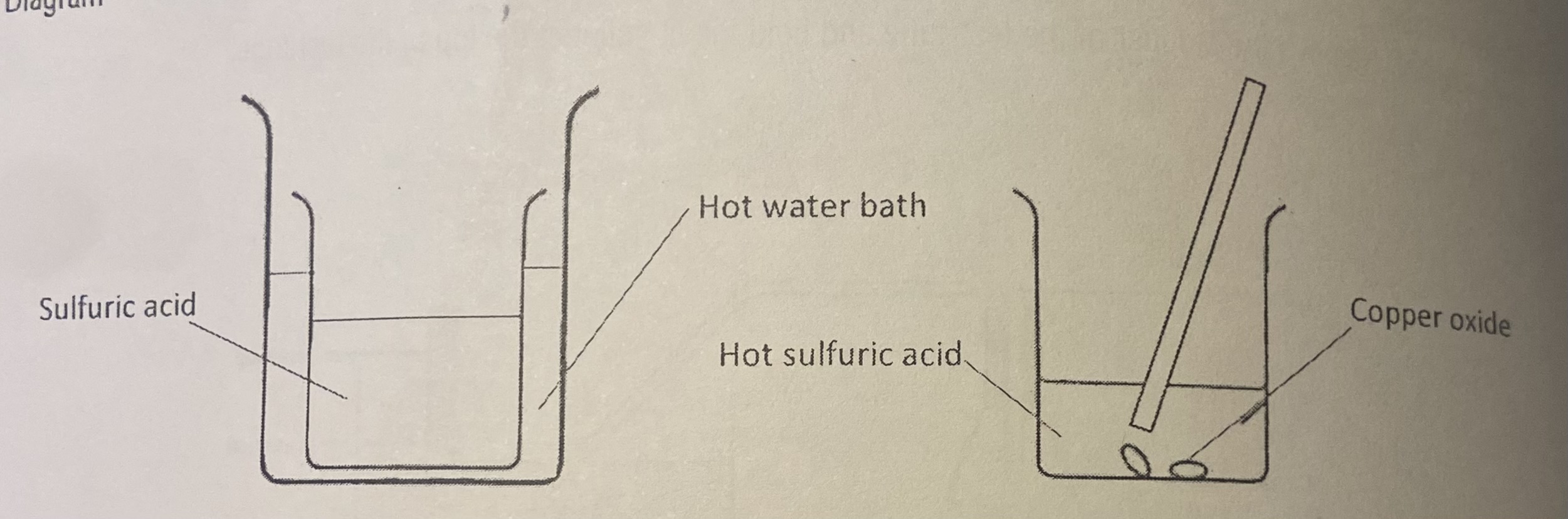
Using a measuring cylinder, measure out 25 cm° of sulfuric acid into the small beaker
Collect approximately 2 g of copper(lI) oxide on a watch glass
Using hot water from a kettle, fill 1/3 of the larger beaker with hot water.
Warm the sulfuric acid beaker by letting it rest (carefully) in the hot water bath (leave for 2 min).
Carefully remove the small beaker and add copper(Il) oxide to the acid slowly, stir with a glass rod
Keep adding the copper(lI) oxide, until there is some left over at the bottom of the beaker. Let the beaker sit for 2 min to allow the black powder to settle.
observation
solution changes from colourless to a pale blue and the copper oxide disappears at the start, but then once all the acid has reaction its a black powder at bottoms
the ph is now slightly acidic