Isotope Practice
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
What isotope has 3 protons, 4 neutrons, and 3 electrons?
Li-3 or Lithium-3
What isotope has 5 protons, 6 neutrons, and 5 electrons?
B-11 or Boron-11
What isotope has 82 protons, 125 neutrons, and 82 electrons?
Pb-207 or Lead 207
What isotope has 12 protons, 13 neutrons, and 12 electrons?
Mg-25 or Magnesium-25
What isotope has 26 protons, 30 neutrons, and 26 electrons?
Fe-56 or Iron-56
What isotope has 17 protons, 18 neutrons, and 17 electrons?
Cl-35 or Chlorine-35
What isotope has 17 protons, 19 neutrons, and 17 electrons?
Cl-36 or Chlorine-36
What isotope has 17 protons, 20 neutrons, and 17 electrons?
Cl-37 or Chlorine-37
What isotope has 29 protons, 56 neutrons, and 20 electrons?
Cu-85 or Copper-85
What isotope has 20 protons, 20 neutrons, and 20 electrons?
Ca-40 or Calcium-40
What is the mass # of Lithium-7?
7
What is the mass # of Magnesium-25?
25
What is the mass # of Chlorine-36?
36
What is the mass # of Chlorine-35?
35
What is the mass # of Lead-207?
207
What is the mass # of Iron-56?
56
What is the mass # of Boron-11?
11
How many protons does Boron-11 have?
5 (it's the atomic number, remember?)
How many protons does Chlorine-37 have?
17 (it's the atomic number, remember?)
How many protons does Calcium-40 have?
20 (it's the atomic number, remember?)
How many protons does Nitrogen-13 have?
7 (it's the atomic number, remember?)
How many protons does Silver-108 have?
47 (it's the atomic number, remember?)
What is the mass number, number of protons, and number of neutrons of 7/3 Li ?
mass number = 7
protons = 3
neutrons = 4
What is the mass number, number of protons, and number of neutrons of 11/5 B ?
mass number = 11
protons = 5
neutrons = 6
What is the mass number, number of protons, and number of neutrons of 13/7 N ?
mass number = 13
protons = 7
neutrons = 6
What is the mass number, number of protons, and number of neutrons of 40/20 Ca ?
mass number = 40
protons = 20
neutrons = 20
What is the mass number, number of protons, and number of neutrons of 85/29 Cu ?
mass number = 85
protons = 29
neutrons = 56
Isotope
any of two or more versions of a chemical element, having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic masses
Mass
The name of an isotope is determined by the atomic __________ of that isotope.
3
The atomic mass of Helium-3 is ___.
4
The atomic mass of Helium-4 is ___.
Lithium
These are isotopes of the element ____________.
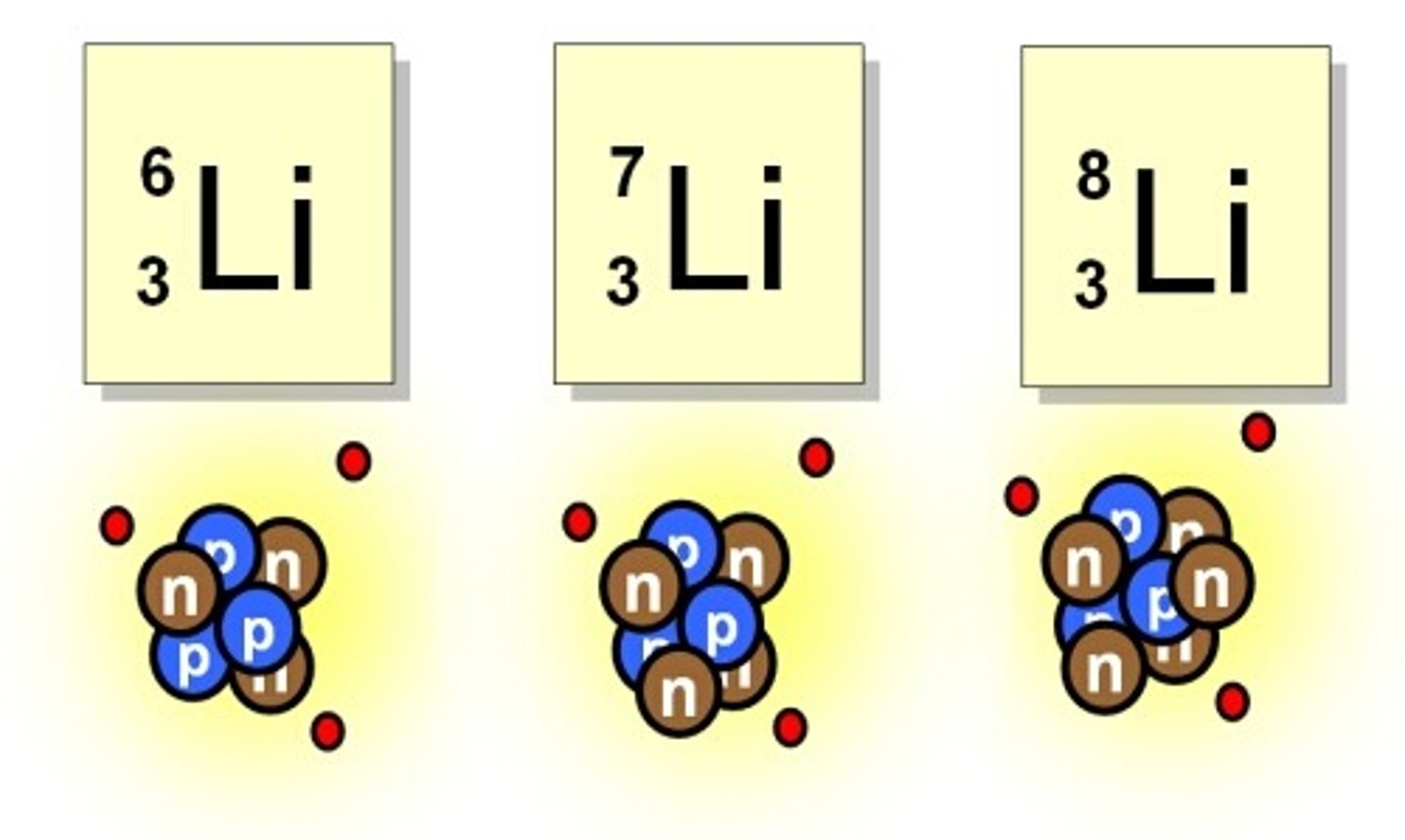
Average
The atomic mass number on the periodic table is the ____________ atomic mass of all isotopes of that element.
5
How many neutrons are in an atom of Lithium-8?
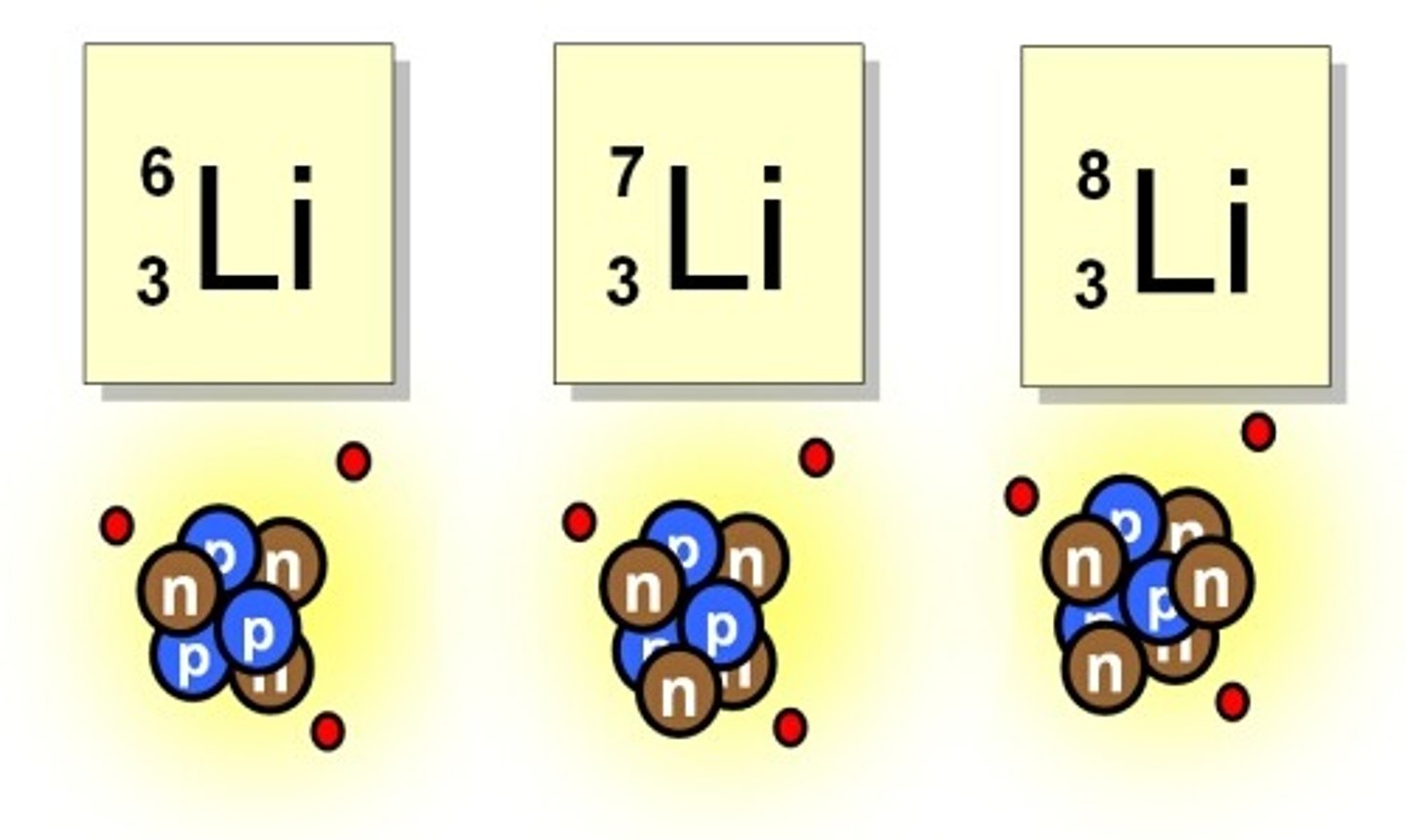
neutrons
The atomic mass of an isotope is determined by adding together the number of protons and the number of ________________.
35
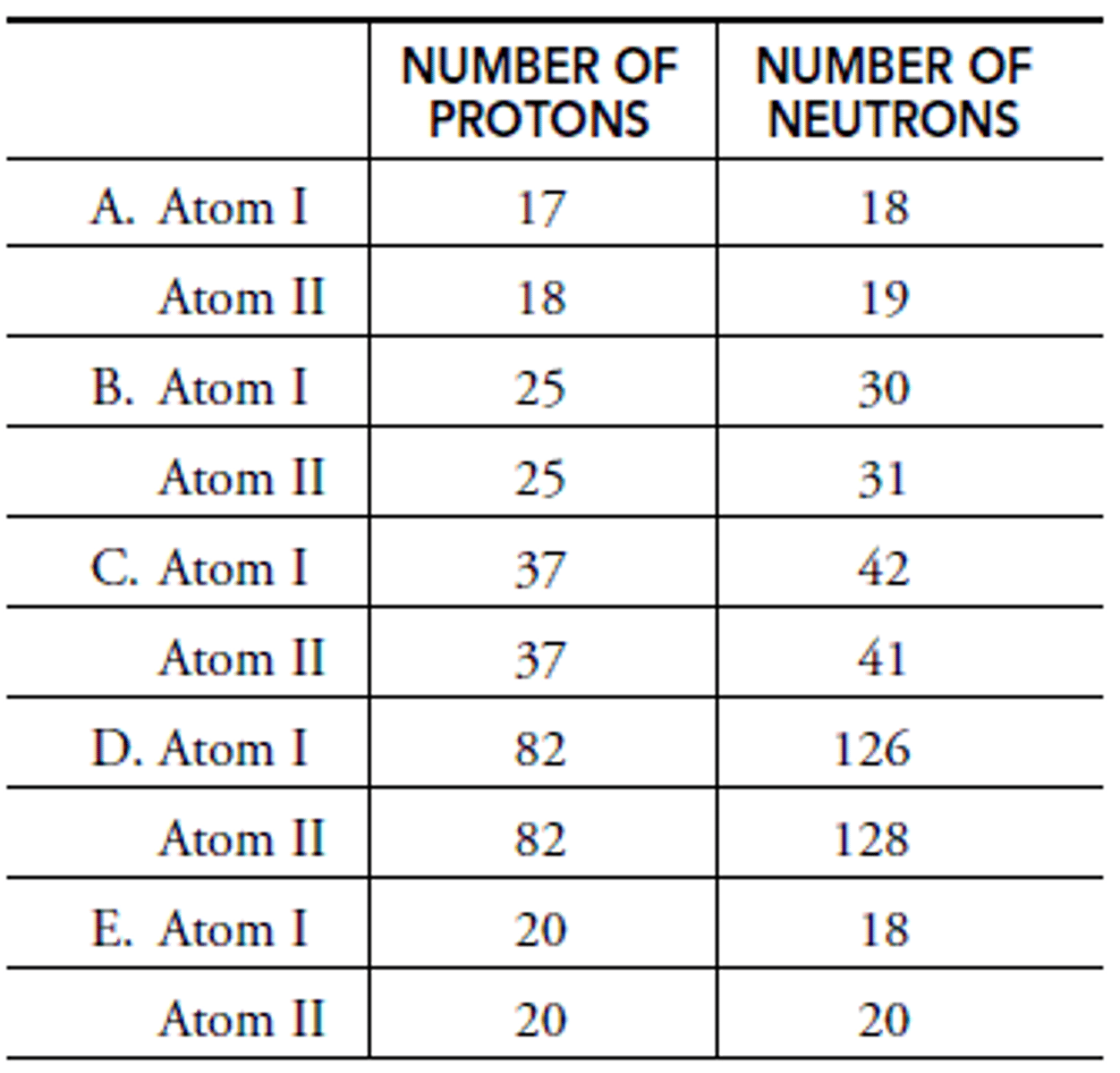
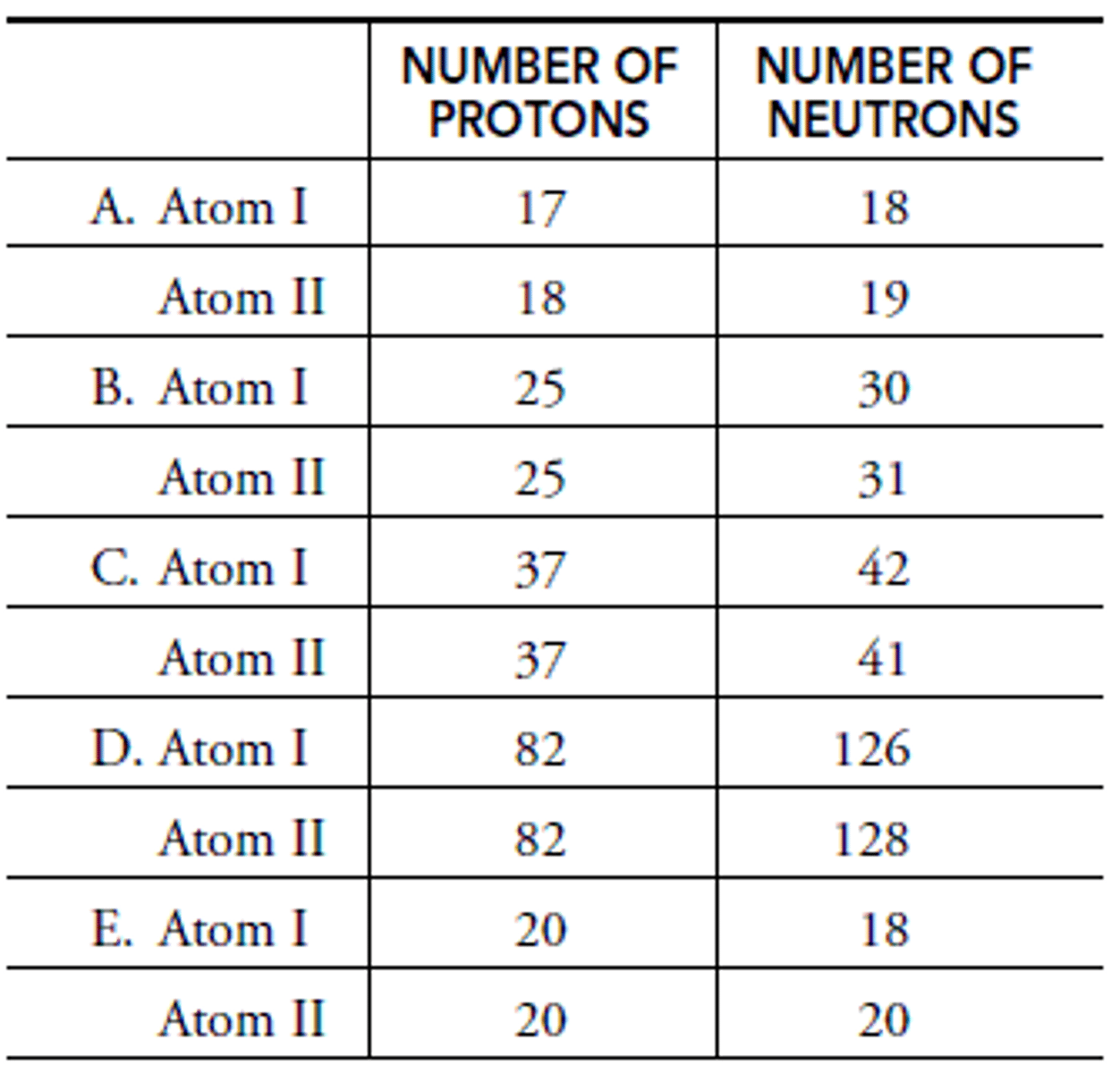
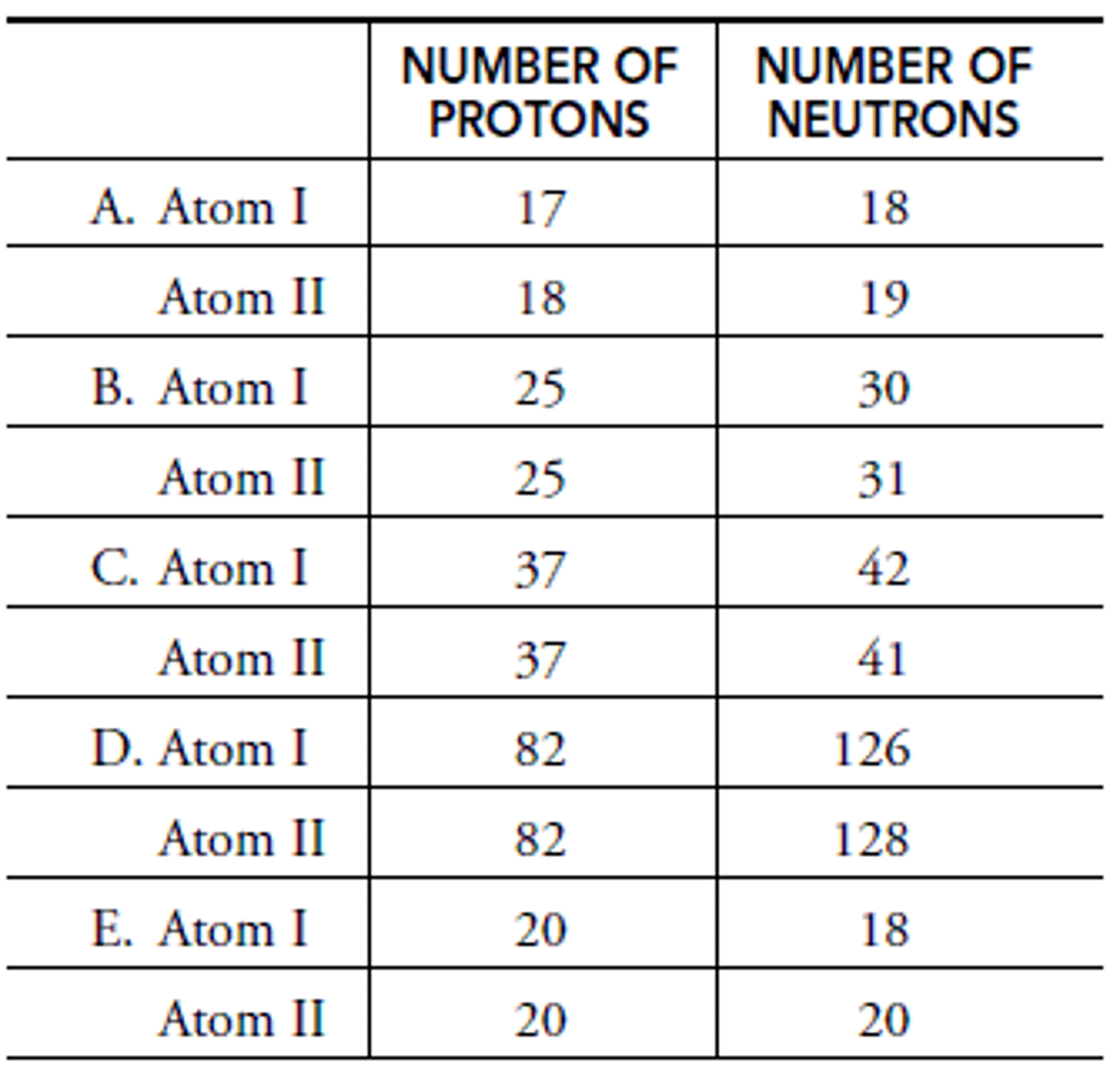
In A: What is the mass of Atom I?
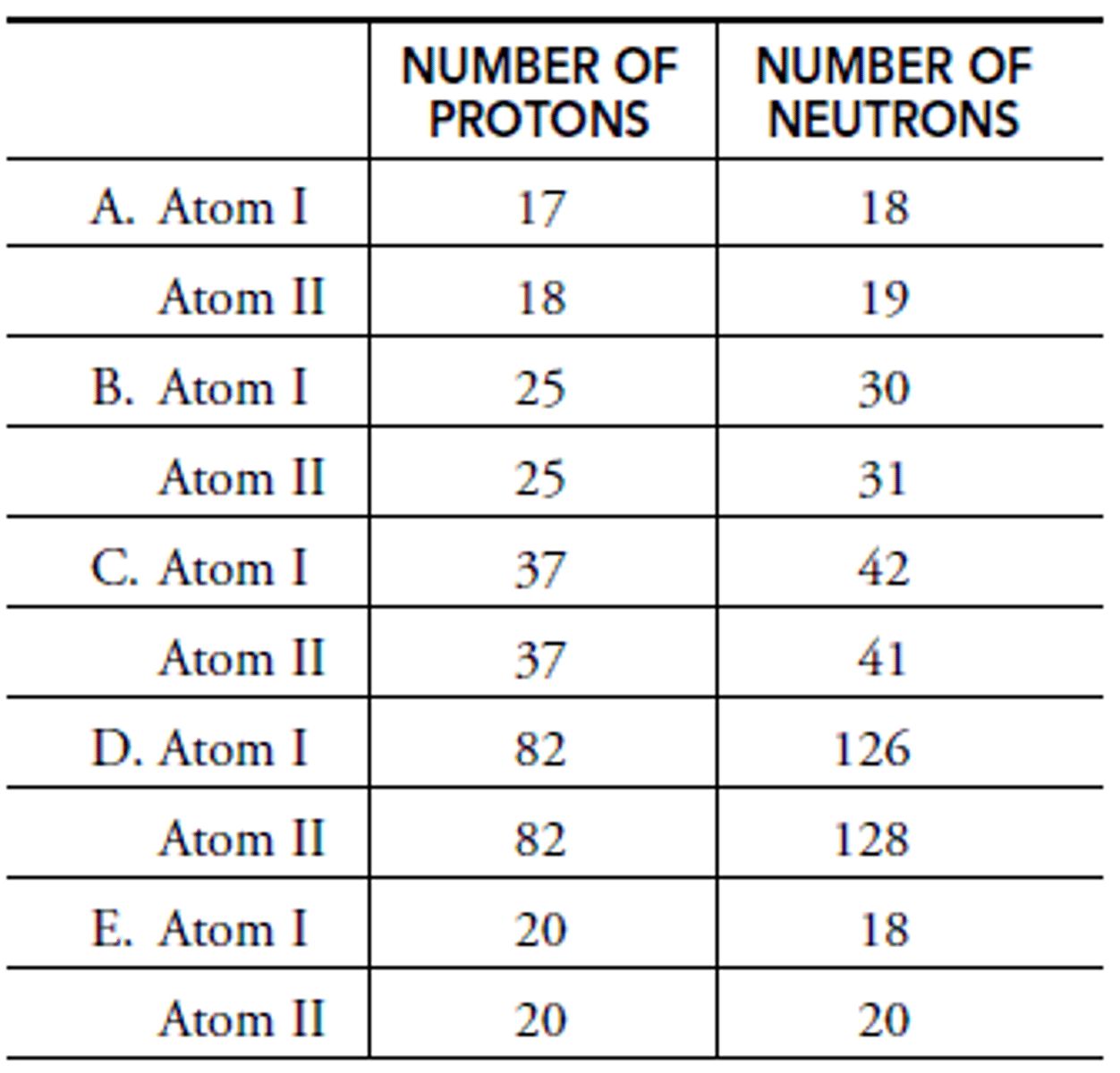
Chlorine
In A: What is the element of Atom I?
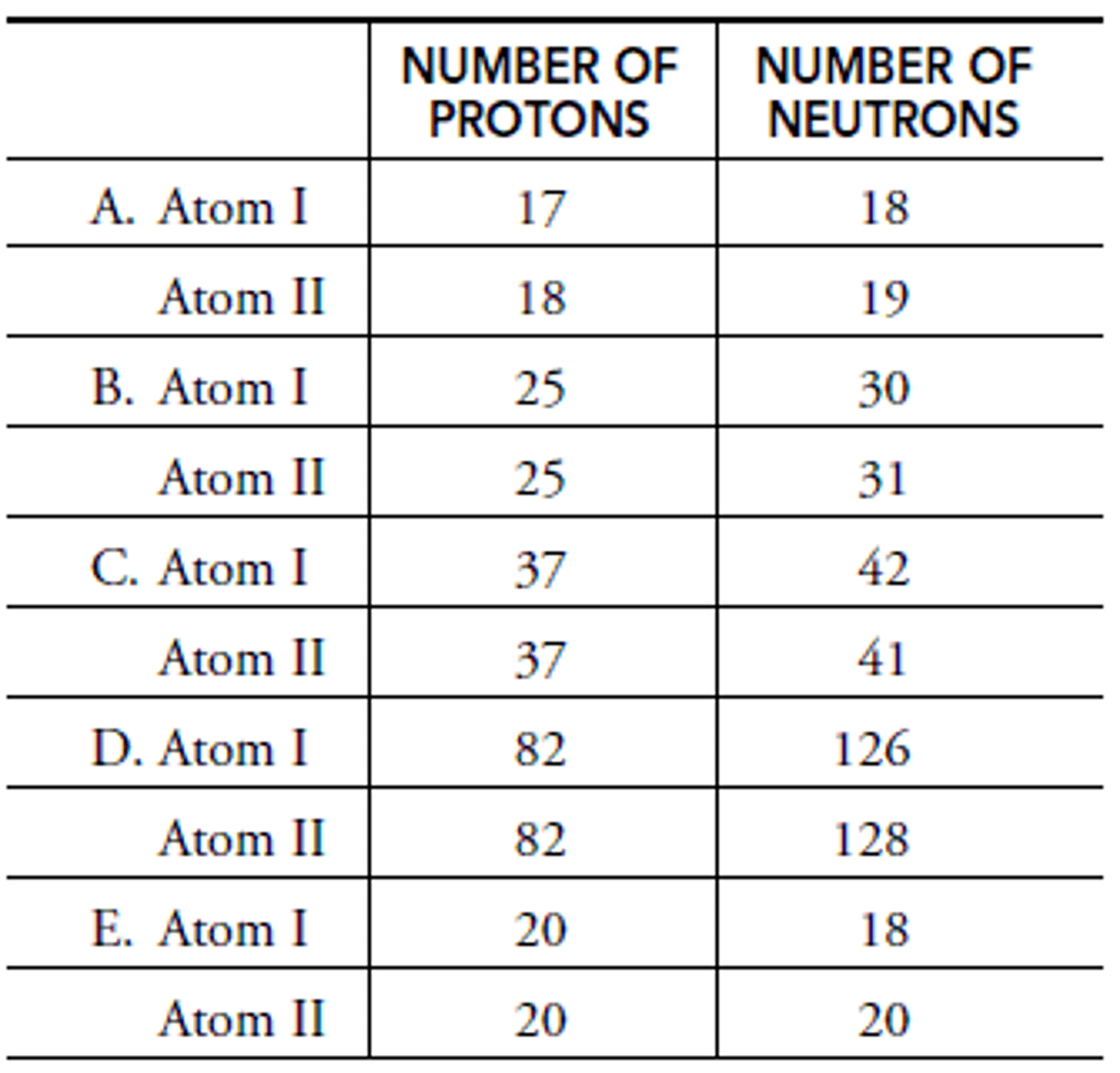
Chlorine-35
In A: What is the isotope name of Atom I?
Argon
In A: What is the element name of Atom II?
Argon-37
In A: What is the isotope name of Atom II?
47
What is the number of the subscript in this isotope of silver?
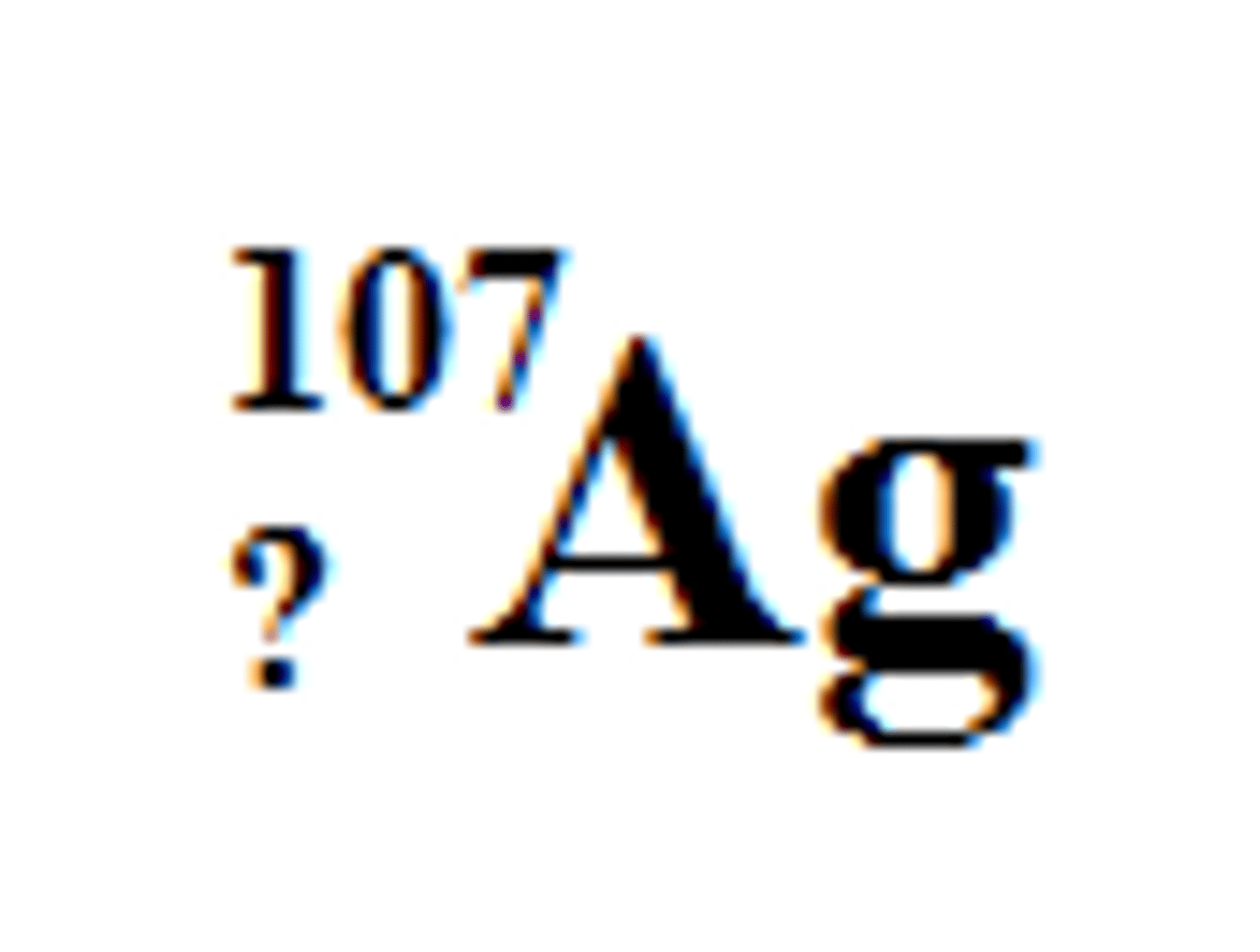
60
How many neutrons are in this isotope?
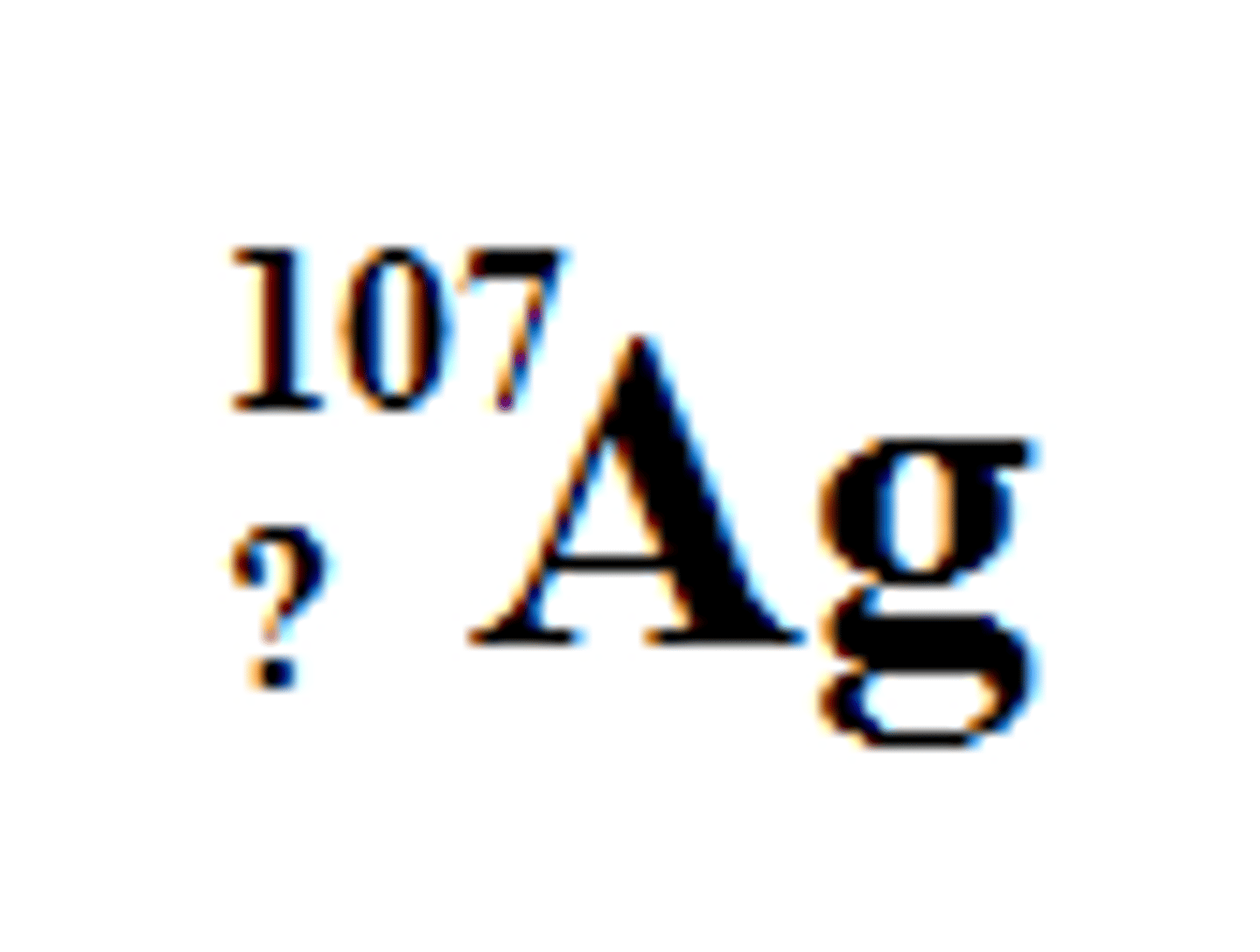
13
What is the mass number of this isotope?
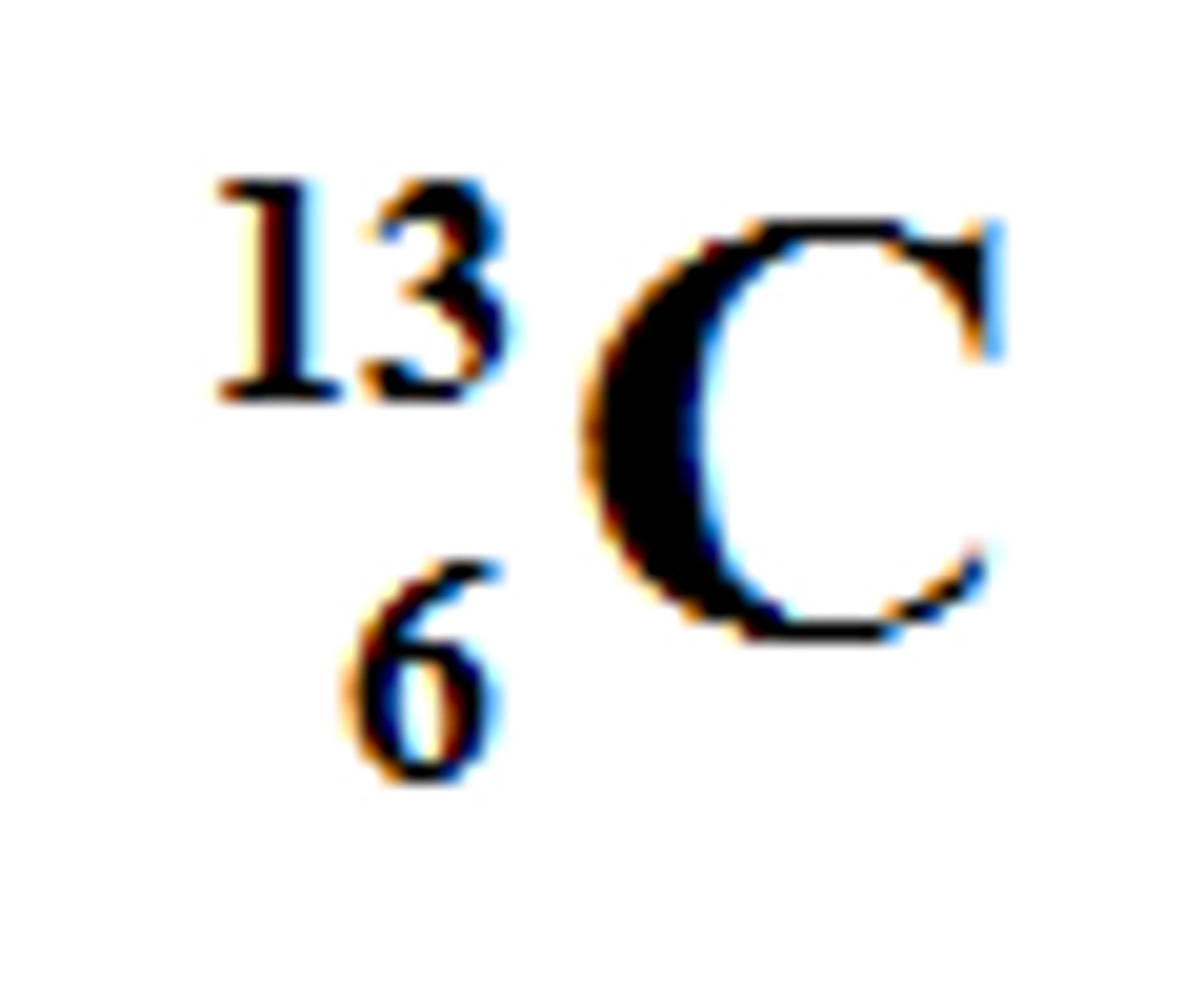
6
How many protons are in this isotope of Carbon?
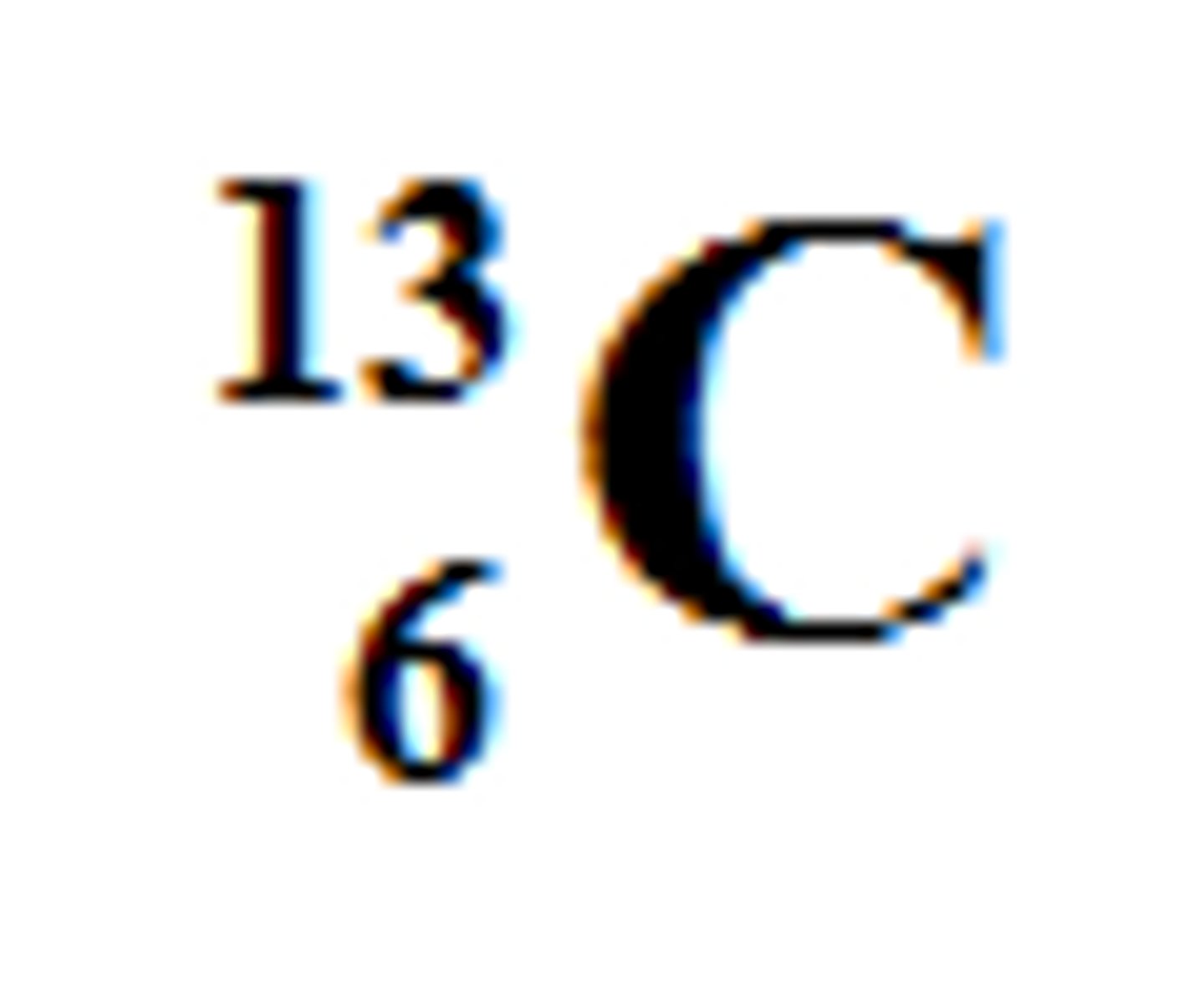
7
How many neutrons are in this isotope of Carbon?
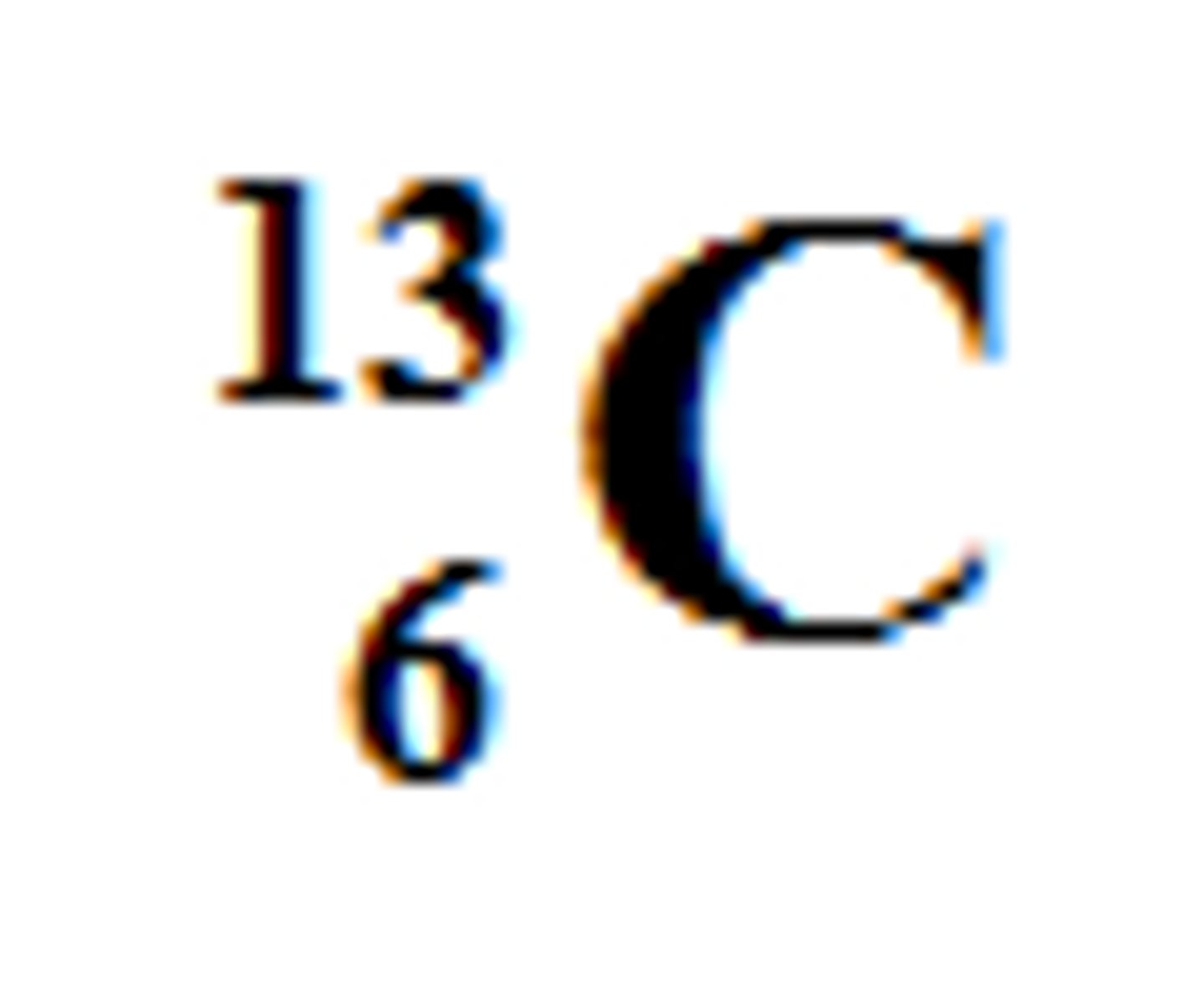
12
How many neutrons are present in Magnesium-24?
25
What is the atomic mass of Magnesium-25?