Properties of Water (1)
1/12
Earn XP
Description and Tags
These flashcards cover the unique properties of water and their significance in biology.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms

What is the molecular shape of water?
Water has a V shape.
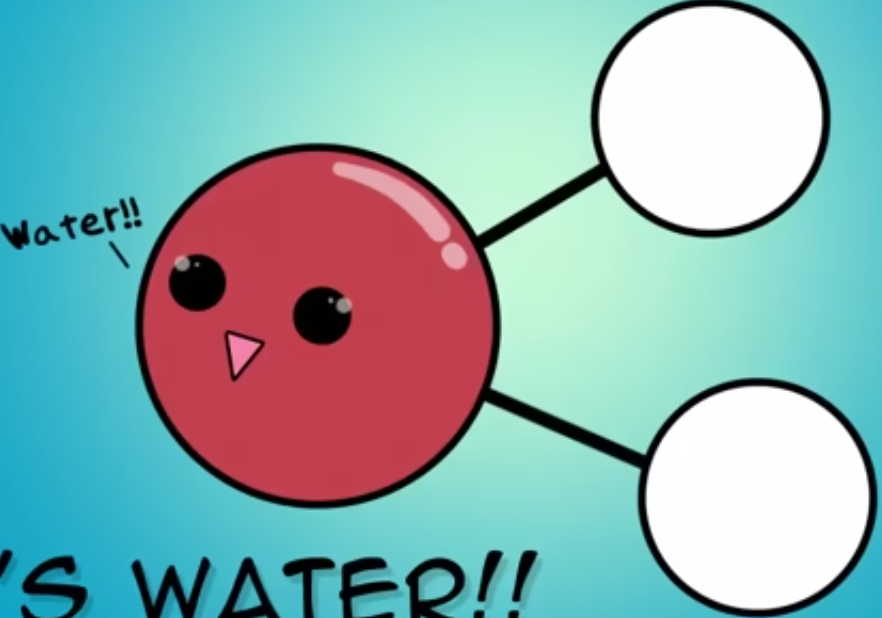
What type of molecule is water?
Water is a polar molecule.
Why do water molecules bond together?
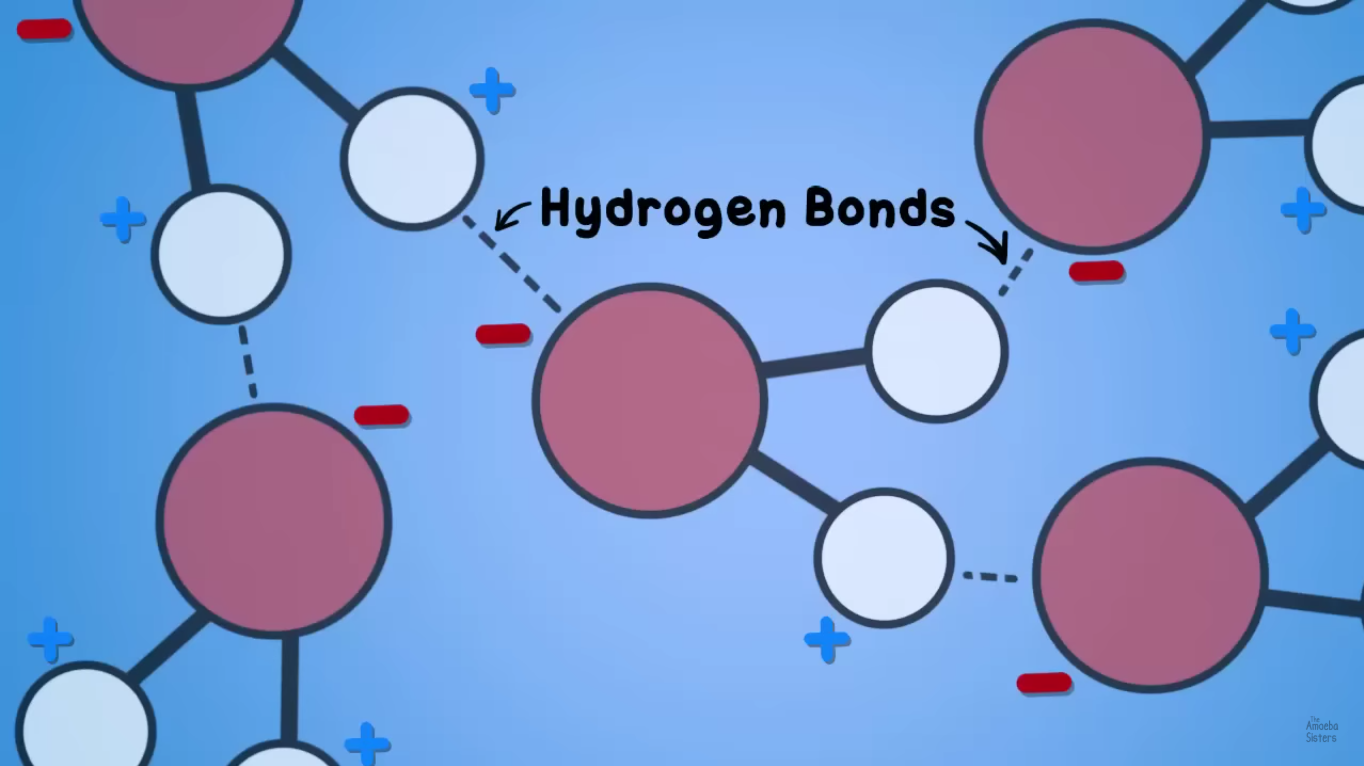
Because the hydrogen of one water molecule has a slightly positive charge and can bond with the slightly negative charge of another water molecule's oxygen.
What are the bonds that form between water molecules called?
Hydrogen bonds.

What is adhesion in the context of water?
The ability of water to stick to the walls of xylem vessels in plants, helping it fight gravity.

What is the phenomenon that allows water molecules to attract each other called?
Cohesion.
How does cohesion contribute to the behavior of water striders?
Cohesion creates surface tension, allowing water striders to skate on the surface.
Why is water considered a powerful solvent?
Because it can dissolve many polar molecules and ionic compounds.
What happens to many substances when they freeze, and how is water different?
Many substances contract and become denser when frozen, but water expands and becomes less dense when frozen.
What is high specific heat in relation to water?
It is the amount of heat needed to raise the temperature of one gram of substance by one degree Celsius, allowing water to stabilize temperatures.
What mechanism do animals and plants use to cool down in hot temperatures?
Evaporative cooling.
What happens to high-energy water molecules during evaporation?
They are the ones most likely to escape into gas, allowing for cooling.
Why does ice float on water?
Because ice is less dense than liquid water due to the structure of hydrogen bonds.