Ganong Chapter 39: Acidification of the Urine and Bicarbonate Excretion
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
Where in the tubule is hydrogen secreted?
The cells of the proximal and distal tubules and in the collecting ducts
What is the transporter responsible for H+ secretion in the proximal tubules?
Na-H exchanger (primary NHE3)
What type of transport occurs at the Na-H exchanger in the proximal tubule?
Secondary active transport
H+ Secretion in the Proximal Tubule
Na+ is moved from the inside of the cell to the interstitium by Na, K ATPase on the basolateral membrane, which keeps intracellular Na+ low, establishing drive for Na+ to enter the cell, via the Na-H exchanger, from the tubular lumen
The Na-H exchanger secretes H+ into the lumen in exchange for Na+
Secreted H+ combines with filtered HCO3- to form H2CO3
The presence of carbonic anhydrase on the apical membrane of the proximal tubules catalyzes the formation of H2O and CO2 from H2CO3
The apical membrane of epithelial cells lining the proximal tubules is permeable to CO2 and H2O and they enter the tubule rapidly
80% of the filtered load of HCO3- is reabsorbed in the proximal tubule
Inside the cell, carbonic anhydrase is also present and can catalyze the formation of H2CO3 from CO2 and H2O
H2CO3 dissociates into H+ ions and HCO3-
H+ is secreted into the tubular lumen and the HCO3- that is formed diffuses into the interstitial fluid
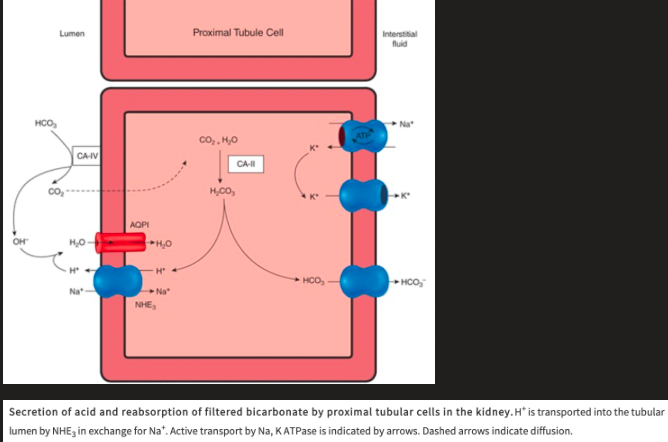
What enters the interstitial fluid for each H+ secreted?
One Na+ and one HCO3-
Action of Carbonic Anhydrase Inhibitors on Acid Secretion in the Proximal Tubule
Because carbonic anhydrase catalyzes the formation of H2CO3, drugs that inhibit carbonic anhydrase depress both secretion of acid by the proximal tubules and the reactions that depend on it
H+ Secretion in the Distal Tubules and Collecting Ducts
In the distal tubules and collecting ducts, H+ secretion is relatively independent of Na+ in the tubular lumen
Most H+ is secreted by an ATP-driven proton pump
Aldosterone acts on this pump to increase distal H+ secretion
The I cells in this part of the renal tubule secrete acid and contain abundant carbonic anhydrase and numerous tubulovesicular structures
Evidence that the H+ translocating ATPase that produces H+ secretion is located in these vesicles as well as in the apical cell membrane
In acidosis the number of H+ pumps is increased by insertion of these tubulovesicles into the apical cell membrane
Some of the H+ is secreted by H-K+ ATPase
The I cells also contain anion exchanger 1 (AE1), an anion exchange protein, in their basolateral cell membranes
May function as a Cl/HCO3 exchanger for the transport of HCO3- to the interstitial fluid
Limiting pH
pH that creates the maximal H+ gradient against which the transport mechanisms can secreted
Normally reached in the collecting ducts
What are the three reactions that occur in the tubular fluid that remove free H+, permitting more acid to be secreted?
Reaction of H+ with HCO3- to form CO2 and H2O
Reaction of HPO42- to form H2PO4- (titratable acids)
Reaction of NH3 to form NH4
Digram of Titratable Acid and Ammonium Formation
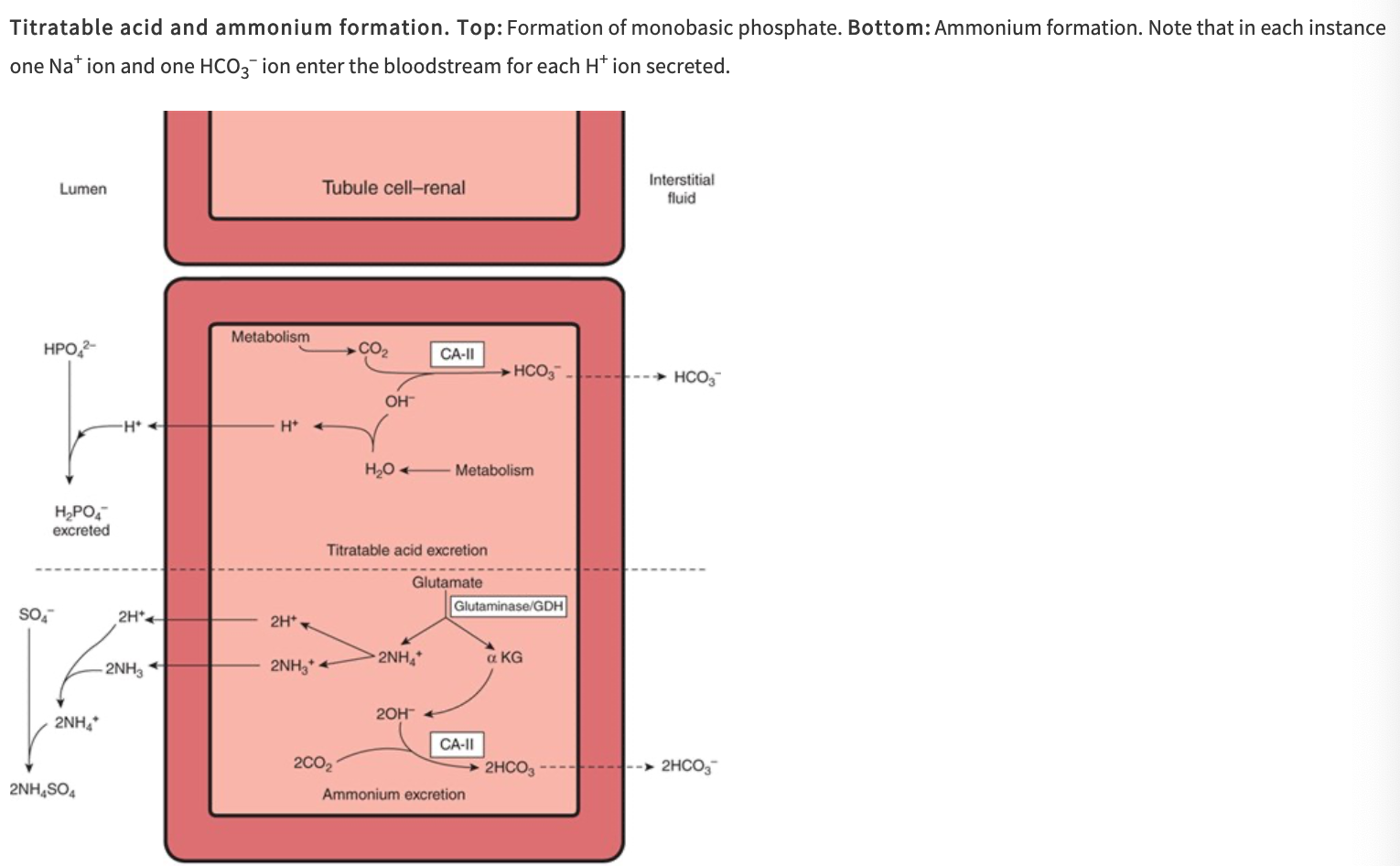
What are the three buffers of importance in the renal handling of acid and its secretion into the lumen?
Bicarbonate
Dibasic phosphate
Ammonia
What % of nonvolatile acids is excreted as titratable acid (phosphate system)?
40%
What % of nonvolatile acids is excreted as NH4+?
60%
Reaction of H+ with HCO3- to form CO2 and H2O
In the proximal tubule, most of the secreted H+ reacts with HCO3- to form H2CO3
This enters the cell as CO2 and H2O following the action of carbonic anhydrase in the brush border of the proximal tubule cells
The CO2 entering the tubular cells adds to the pool of CO2 available to form H2CO3
Most of the H+ is removed from the tubule so the pH of the fluid is changed very little
This is the mechanism by which HCO3- is reabsorbed; for each mole of HCO3- removed from tubular fluid, 1 mol of HCO3- diffuses from the tubular cells into the blood
Reaction of HPO42- to form H2PO4- (titratable acids)
Secreted H+ reacts with dibasic phosphate (HPO42-) to form monobasic phosphate (H2PO4-)
Happens to the greatest extent in the distal tubules and collecting ducts, because this is where the phosphate that escapes proximal reabsorption is greatly concentrated by the reabsorption of water
Ammonia Buffering System
The ammonia buffering system allows secreted H+ to combine with NH3
Occurs in the proximal tubule (where NH3 is made) and in the distal tubules
The pK' of the ammonia system is 9.0 and the ammonia system is titrated only from the pH of the urine to pH 7.4 so it contributes very little to the titratable acidity
Each H+ that reacts with the buffers contributes to the urinary titratable acidity which is measured by determining the amount of alkali that must be added to the urine to return its pH to 7.4, the pH of the glomerular filtrate
The titratable acidity measures only a fraction of the acid secreted since it does not account for the H2CO3 that has been converted to H2O and CO2
Ammonia Secretion
Reactions in the renal tubular cells produce NH4+ and HCO3-
NH4+ is in equilibrium with NH3 and H+ in the cells
The pK' of this reaction is 9.0 so the ratio of NH3 to NH4+ at pH 7.0 is 1:100
NH3 is lipid-soluble and diffuses across the cell membranes down its concentration gradient into the interstitial fluid and tubular urine
In the urine it reacts with H+ to form NH4+ and the NH4+ remains "trapped" in the urine
What are the reactions producing NH4+ in cells?
Principally, conversion of glutamine to glutamate
Catalyzed by the enzyme glutaminase, which is abundant in renal tubular cells
Glutamate dehydrogenase catalyzes the conversion of glutamate to a-ketoglutarate, with the production of more NH4+
Subsequent metabolism of a-ketoglutarate utilizes 2H+, freeing 2HCO3-
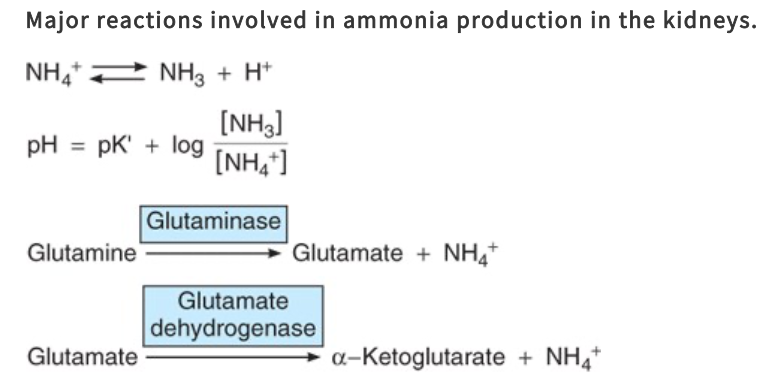
Ammonia Excretion in Chronic Acidosis
In chronic acidosis, the amount of NH4+ excreted at any given urine pH also increases, because more NH3 enters the tubular urine
The effect of this adaption of NH3 secretion is further removal of H+ from the tubular fluid and consequently a further enhancement of H+ secretion by the renal tubules and excretion in the urine
How can the kidneys remove the normal amount of even an increased amount of nonvolatile acid produced in the body?
The production of NH4+ by the renal tubules is the only way the kidneys can remove even the normal amount, much less an increased amount of nonvolatile acid produced in the body
The amount of phosphate buffer filtered at the glomerulus cannot be increased, so urinary excretion of acid via the phosphate buffer system is limited
What is the main process by which NH3 is secreted into the urine and changed to NH4+ in the inner medullary cells of the collecting duct?
Nonionic diffusion
Maintains the concentration gradient for diffusion of NH3
Salicylates and other drugs that are weak bases or weak acids are also secreted by nonionic diffusion
They diffuse into the tubular fluid at a rate that depends on the pH of the urine
Nonionic Diffusion in the Proximal Tubule
In the proximal tubule, nonionic diffusion of NH4+ is less important because NH4+ can be secreted into the lumen, often by replacing H+ on the Na-H exchanger
pH Changes Along the Nephron
A moderate drop in pH occurs in the proximal tubular fluid, but most of the secreted H+ has little effect on luminal pH because of the formation of CO2 and H2O from H2CO3
The distal tubule has less capacity to secrete H+, but secretion in this segment has a greater effect on urinary pH
What factors alter renal acid secretion?
Change in the intracellular PCO2
K+ Concentration
Carbonic anhydrase level
Adrenocortical hormone concentration
Alterations in Renal Acid Secretion from Changes in the Intracellular PCO2
When the PCO2 is high (respiratory acidosis), more intracellular H2CO3 is available to buffer the hydroxyl ions and acid secretion is enhanced
The reverse is true when PCO2 falls
Alterations in Renal Acid Secretion from K+ Concentration
K+ depletion enhances acid secretion because the loss of K+ causes intracellular acidosis even though the plasma pH may be elevated
K+ excess inhibits acid secretion
Alterations in Renal Acid Secretion from Carbonic Anhydrase Level
When carbonic anhydrase is inhibited, acid secretion is inhibited because the formation of H2CO3 is decreased
Alterations in Renal Acid Secretion from Adrenocortical Hormone Concentration
Aldosterone and the other adrenocortical steroids that enhance tubular reabsorption of Na+ also increase the secretion of H+ and K+
Bicarbonate Excretion
The process of HCO3- reabsorption does not involve actual transport of this ion into the tubular cells but HCO3- reabsorption is proportional to the amount filtered over a relatively wide range
HCO3- reabsorption is decreased by an unknown mechanism when the ECF volume is expanded
When the plasma HCO3- concentration is low, all the filtered HCO3- is reabsorbed
The lower the plasma HCO3- concentration drops, the more acidic the urine becomes and the greater its NH4+ content
When the plasma HCO3- concentration is high, HCO3- appears in the urine and the urine becomes alkaline
What is the core of acid base balance?
Maintenance of the H+ concentration of the ECF
How can intracellular H+ concentration be measured?
Using microelectrodes, pH-sensitive fluorescent dyes, and phosphorous magnetic resonance
Distinct from extracellular pH but is sensitive to changes in ECF H+ concentration
What does a decrease in pH of 1 unit represent?
A 10 fold increase in H+ concentration
pH of True Plasma
pH of blood is the pH of true plasma because red cells contain hemoglobin, which is quantitatively one of the most important blood buffers
True plasma - plasma that has been in equilibrium with red cells
pH of Arterial Plasma vs Venous Plasma
pH of arterial plasma is normally 7.40 and venous plasma is slightly lower
When is acidosis present?
Whenever the arterial pH is below 7.40
When is alkalosis present?
Whenever pH is above 7.40
H+ Concentrations in the ECF that are Compatible with Life
The H+ concentrations in the ECF that are compatible with life cover an approximately fivefold range, from 0.00002 mEq/L (pH 7.70) to 0.0001 mEq/L (pH 7.00)
Role of the Liver and Kidneys in the Handling of Metabolically Produced Acid Loads
Amino acids are utilized in the liver for gluconeogenesis, leaving NH4+ and HCO3- as products from their amino and carboxyl groups
NH4+ is incorporated into urea and the protons that are formed are buffered intracellularly by HCO3-, so little NH4 and HCO3- escape into circulation
Metabolism of sulfur-containing amino acids produces H2SO4 and metabolism of phosphorylated amino acids such as phosphoserine produces H3PO4
These strong acids enter the circulation and present a major H+ load to the buffers in the ECF
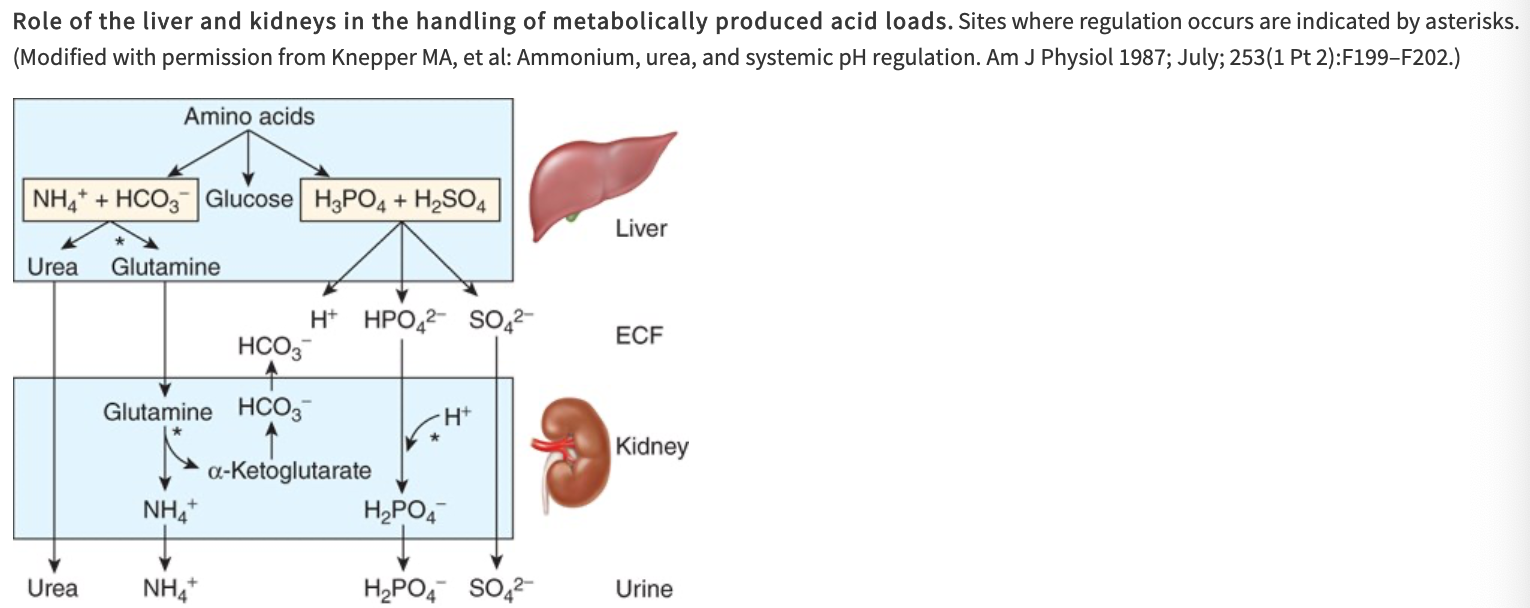
What happens to CO2 formed by metabolism in the tissues?
It is in large part hydrated to H2CO3
Most of the CO2 is excreted in the lungs
Only small quantities of the H+ remain to be excreted by the kidneys
Common Sources of Extra Acid Loads
Strenuous exercise (lactic acid)
Diabetic ketosis (acetoacetic acid and B-hydroxybutyric acid)
Ingestion of acidifying salts such as NH4Cl and CaCl2, which in effect add HCl to the body
Failure of diseased kidneys to excrete normal amounts of acid is another cause of acidosis
Causes of Alkalosis
Main source of alkali is from ingested Na+ and K+ salts of weak organic acids, and the anions of these salts are metabolized to CO2, leaving NaHCO3 and KHCO3 in the body
Alkalinizing salts are sometimes ingested in large amounts
A more common cause of alkalosis is loss of acid from the body as a result of vomiting of gastric juice rich in HCl
Where is carbonic anhydrase found in high concentrations?
In gastric acid secreting cells and in renal tubular cells
What inhibits carbonic anhydrase?
Cyanide
Azide
Sulfide
Principal Buffers in the Blood

Principal Buffers in Interstitial Fluid

Principal Buffers in Intracellular Fluid

What are the principal buffers in the CSF and urine?
Bicarbonate and phosphate systems
Where is the acid load buffered in metabolic acidosis?
Only 15-20% of the acid load is buffered by the H2CO3-HCO3- system in the ECF and most of the remainder is buffered in cells
What % of the OH-load is buffered in cells in metabolic alkalosis?
30-35%
Where does buffering occur in respiratory acidosis and alkalosis?
Almost all of the buffering is intracellular
What are the principal regulators of intracellular pH in animal cells?
HCO3- transporters
Include the Cl-HCO3- exchanger AE1, three Na+ - HCO3- cotransporters, and a K+ - HCO3- cotransporter
What are the three buffer anions?
Hb-
Prot-
HCO3-
Renal Compensation to Respiratory Acidosis and Alkalosis
HCO3- reabsorption in the renal tubules depend not only on the filtered load of HCO3-, which is the product of the glomerular filtration rate and the plasma HCO3- level, but also on the rate of H+ secretion by the renal tubular cells because HCO3- is reabsorbed by exchange for H+
The rate of H+ secretion, and therefore HCO3- reabsorption, is proportional to the arterial PCO2
The more CO2 that is available to form H2CO3 in the tubular cells, the more H+ that can be secreted
When the PCO2 is high, the interior of most cells becomes more acidic
Renal Compensation of Respiratory Acidosis
In respiratory acidosis, renal tubular H+ secretion is increased, removing H+ from the body
Even though the plasma HCO3- is elevated, HCO3- reabsorption is increased, further raising the plasma HCO3-
In the shift from acute to chronic respiratory acidosis, Cl- excretion is increased and plasma Cl- falls as plasma HCO3- is increased
Renal Compensation of Respiratory Alkalosis
In respiratory alkalosis, the low PCO2 hinders renal H+ secretion, HCO3- reabsorption is depressed, and HCO3- is excreted, further reducing the already low plasma HCO3- and lowering the pH toward normal
Metabolic Acidosis
When acids stronger than Hb and the other buffer acids are added to blood, metabolic acidosis is produced
For example, if H2SO4 is added, the H+ is buffered and the Hb-, Prot-, and HCO3- levels in plasma drop
The H2CO3 formed is converted to H2O and CO2 and the CO2 is rapidly excreted via the lungs
Uncompensated metabolic acidosis
The rise in plasma H+ stimulates respiration so that the PCO2, instead of rising or remaining constant, is reduced
This respiratory compensation raises the pH even further
Renal compensatory mechanisms then bring about the excretion of the extra H+ and return the buffer systems to normal
Renal Compensation for Acute Metabolic Acidosis
The anions that replace HCO3- in the plasma in metabolic acidosis are filtered, each with a cation (principally Na+), maintaining electrical neutrality
Renal tubular cells secrete H+ into the tubular fluid in exchange for Na+
For each H+ secreted, one Na+ and one HCO3- are added to the blood
The limiting urinary pH would be reached rapidly and the total amount of H+ secreted would be small if no buffers were present in the urine to "tie up" H+
Secreted H+ reacts with HCO3- to form CO2 and H2O (bicarbonate reabsorption); with HPO42- to form H2PO4-; and with NH3 to form NH4+
Allows large amounts of HCO3- to be returned to or added to the depleted body stores and large numbers of the cations to be reabsorbed
Only when the acid load is very large that cations are lost with the anions, producing diuresis and depletion of body cation stores
In metabolic acidosis, the respiratory compensation tends to inhibit the renal response in the sense that the induced drop in PCO2 hinders acid secretion, but it also decreases the filtered load of HCO3- and so its net inhibitory effect is not great
Renal Compensation of Chronic Metabolic Acidosis
In chronic acidosis, glutamine synthesis in the liver is increased, using some of the NH4+ that is usually converted to urea
Glutamine provides the kidneys with an additional source of NH4+
NH3 secretion increase over a period of days, further improving the renal compensation for acidosis
Metabolism of glutamine in the kidneys produces a-ketoglutarate which is decarboxylated, producing HCO3-, which enters the bloodstream and helps buffer the acid load
Metabolic Acidosis
In metabolic alkalosis, the plasma HCO3- level and pH rise
Respiratory compensation is a decrease in ventilation produced by the decline in H+ concentration which elevates the PCO2
Brings the pH back toward normal while elevating the plasma HCO3- level further
Magnitude of compensation is limited by the carotid and aortic chemoreceptor mechanisms, which drive the respiratory center if any appreciable fall occurs in the arterial PO2
More renal H+ secretion is expended in reabsorbing the increased filtered load of HCO3-
The rise in PCO2 inhibits the renal compensation by facilitating acid secretion, but its effect is relatively slight
Which of the following is the principal buffer in interstitial fluid?
a. hemoglobin
b. other proteins
c. carbonic acid
d. H2PO4
e. compounds containing histidine
c. carbonic acid
Increasing alveolar ventilation increases blood pH because:
a. it activates neural mechanisms that remove acid from blood
b. it makes hemoglobin a stronger acid
c. it increases the PO2 of the blood
d. it decreases the PCO2 in the alveoli
e. the increased muscle work of increased breathing generates more CO2
d. it decreases the PCO2 in the alveoli
In uncompensated metabolic alkalosis:
a. the plasma pH, the plasma HCO3- concentration, and the arterial PCO2 are all low
b. the plasma pH is high and the plasma HCO3- concentration and arterial PCO2 are low
c. the plasma pH and the plasma HCO3- concentration are low and the arterial PCO2 is normal
d. the plasma pH and the plasma HCO3- concentration are high and the arterial PCO2 is normal
e. the plasma pH is low, the plasma HCO3- concentration is high, and the arterial PCO2 is normal
d. the plasma pH and the plasma HCO3- concentration are high and the arterial PCO3 is normal
In a patient with a plasma pH of 7.10, the [HCO3-]/[H2CO3] ratio in plasma is:
a. 20
b. 10
c. 2
d. 1
e. 0.1
b. 10