2: symmetry and bonding
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
define
symmetry element
symmetry operation
element = geometrical feature i.e. point, axis, plane
operation = act of moving in space w.r.t. element i.e. rotation about an axis, reflection in a plane, inversion in/through a point
what are the 5 kinds of symmetry operations
proper rotations
reflections
identity
improper rotations
inversion
describe proper rotations
C(n)
rotation about an axis by 360/n
there are (n-1) rotations for any C(n) axis
what is the principal axis of rotation
C(n) with highest value of n
describe reflections
σ(v) = reflection plane containing principal axis of rotation
σ(h) = reflection plane perpendicular to principal axis of rotation
σ(d) = reflection plane diagonal to principal axis of rotation
describe identity
E = doing nothing to an object
σ² = i² = E
describe inversion
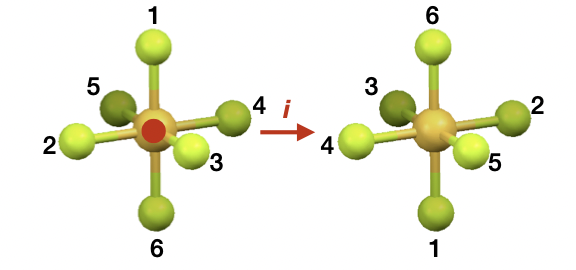
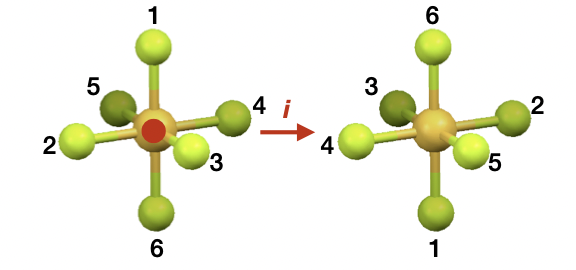
describe improper rotations
composite operation = rotation around principal axis and reflection in σ(h)
describe the process of assigning point groups
principal axis of rotation
perpendicular C2 axis?
yes:
= D
σ(h) = Dnh
σ(d) = Dnd
or Dn
no:
σ(h) = Cnh
σ(v) = Cnv
S2n (improper rotation w.r.t to C2 axis) = S2n
or Cn
σ(h) takes precedent
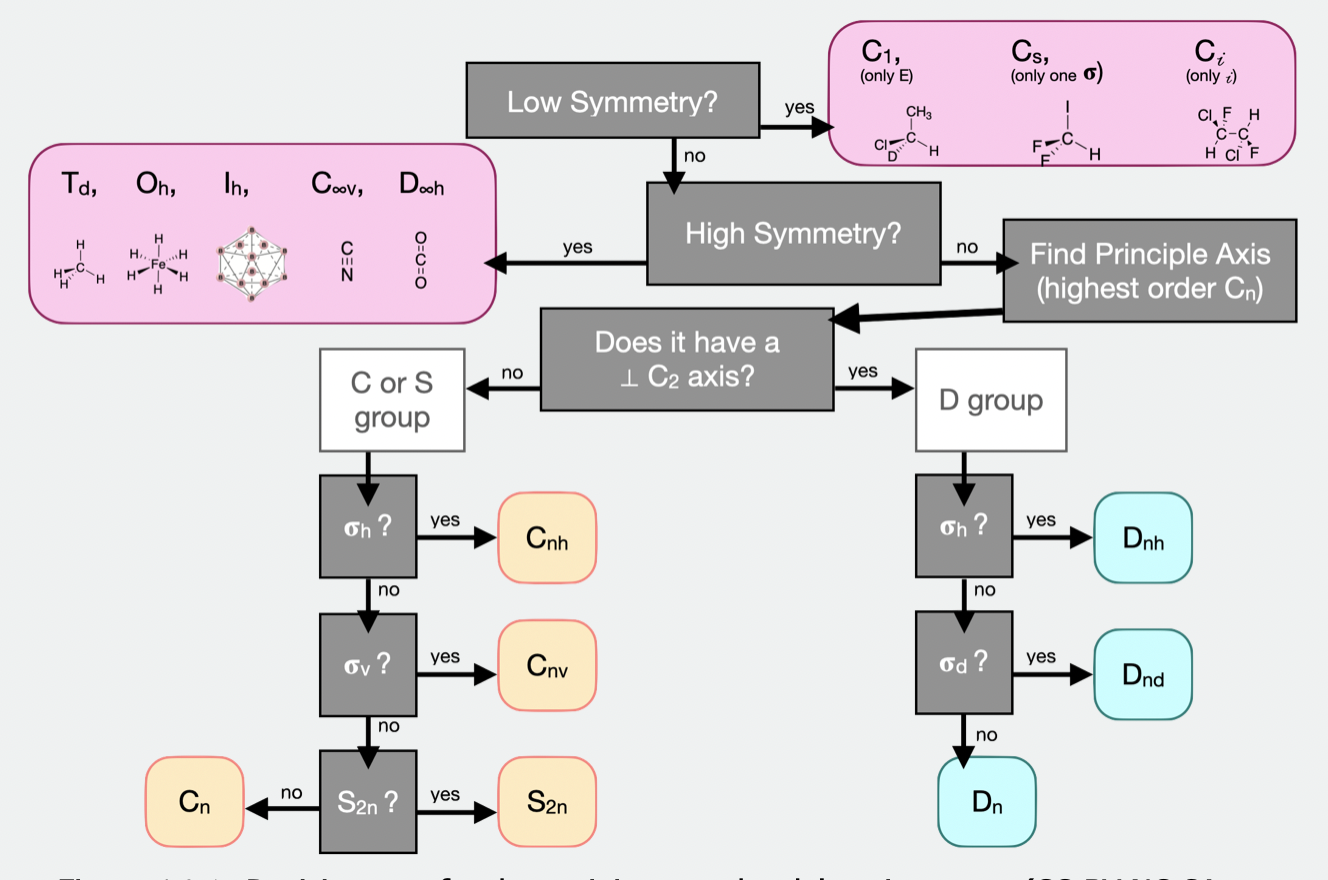
what are the low symmetry point groups?
no symmetry (only E) = C1
only σ = Cs
only i = Ci
what are the high symmetry point groups?
tetrahedral = Td
octahedral = Oh
isohedral = Ih
linear with same end = D∞h
linear with different end = C∞v
what is a group in group theory?
collection of elements that obey certain well-defined criteria
what is a point group w.r.t. group theory?
an objects symmetry operation form a point group, where the group operation “ ° “ is understood to be the successive application of symmetry operations
what is the criteria of group theory?
closure
= results of two elements by an operation is another element
identity
= set includes an identity element which leaves another element unchanged after operation
inverse
= each element has an inverse which returns element to identity after operation
associativity
( A ° B ) ° C = A ( B ° C )
(generally not commutative)
what are group multiplication tables?
to obey the closure criteria, application of two subsequent symmetry operations must be equivalent to a single symmetry operation
form the group multiplication table for C(2v)
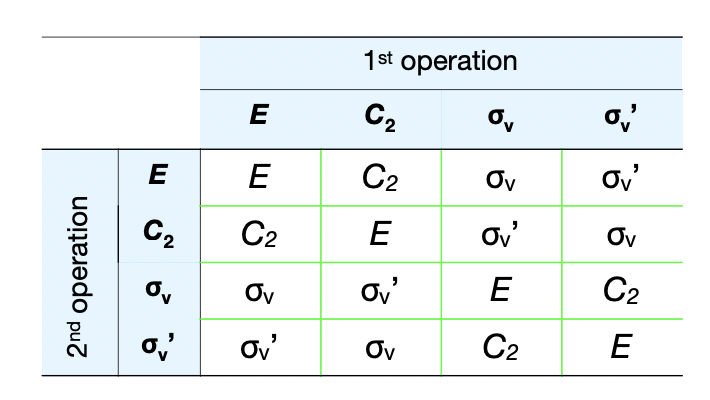
in what order are symmetry operations applied
successive operations are written from right to left i.e. apply operation to whats on its right first
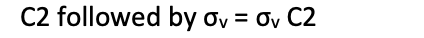
how do wavefunctions have a sign?
they change sign between different regions of space
probability = square of the wavefunction
hence, sign is not observable
what is a character?
= X
= defines how a feature/property (typically an orbital) is affected by a particular symmetry operation
what are the possible characters?
+1 = no change
0 = moves
-1 = no change, sign flip
what is an invariant point?
= point which does not move upon application of symmetry operations
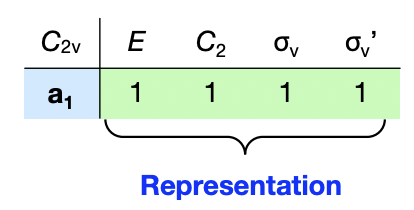
what is a representation?
= Γ
= the complete set of characters for a particular feature/property from application of all symmetry operations
= encapsulates the symmetry behaviour of a feature/property
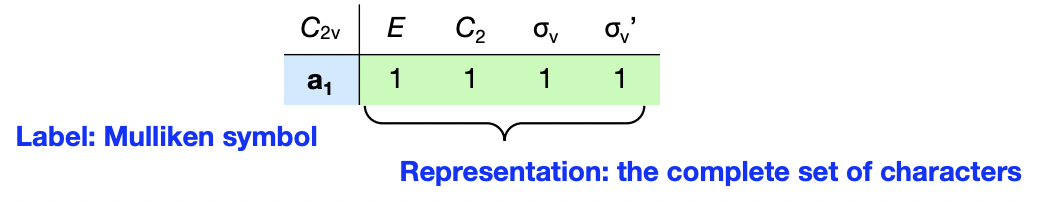
what is a Mulliken symbol
= a label for a (irreducible) representation
how can we describe the symmetry of a particular feature/property?
the ‘feature/property’ on X forms a basis for the ‘Mulliken symbol’ of the ‘point group’
what is a character table?
lists all the irreducible representations of a point group
what is meant by ‘classes of symmetry operations’?
symmetry operations in character table can be multiples = a class of the symmetry operation
the character in the irreducible representation refer to classes of symmetry operations = single character = SUM THE RESULTING CHARACTERS
how can the orbitals represented by a symmetry species be determined?
by the right hand column
describe the linear combination of atomic orbitals (LCAO)
the many electron wavefunction of a molecule is constructed from the one electron wavefunctions
orbital = one electron wavefunction
atomic orbital = one electron wavefunction of an atom
molecule orbital = many electron wavefunction of a molecule
how does wavefunction interference arise?
overlapping of atomic orbitals
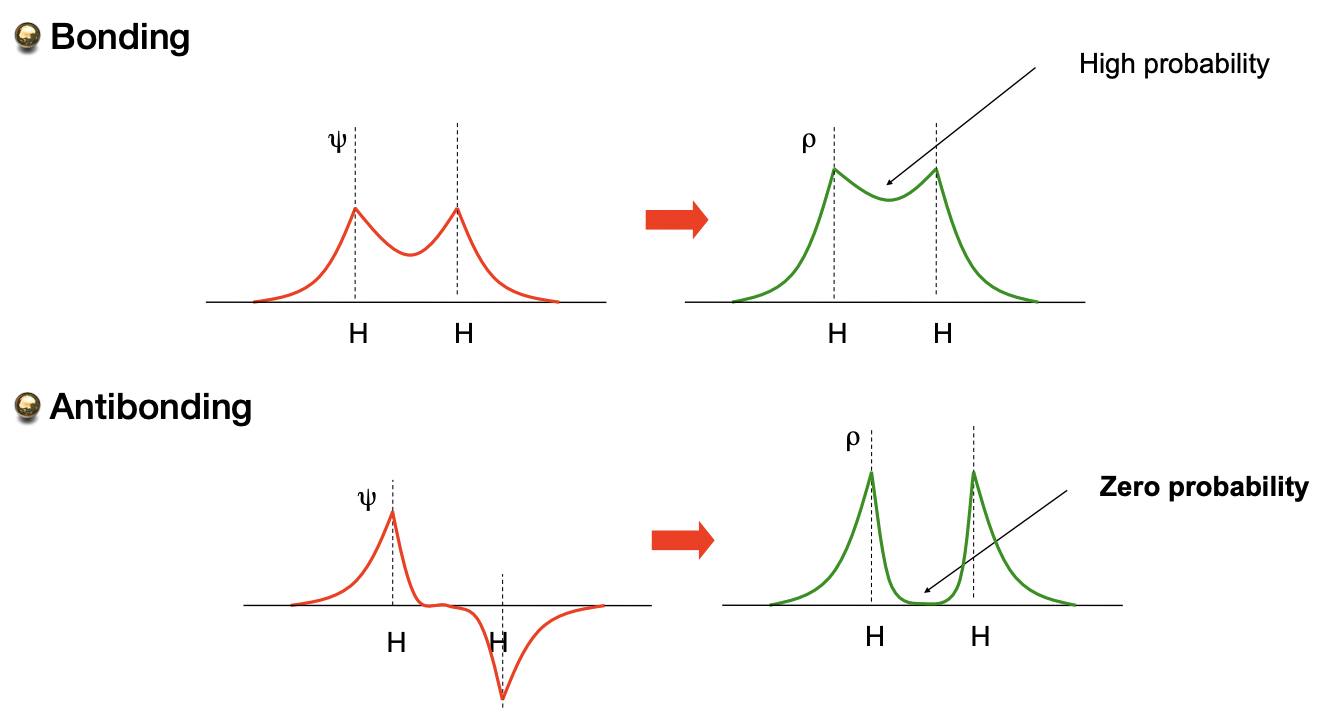
describe the two types of wavefunction interference
constructive = bonding MO
destructive = anti-bonding MO
describe the probability of finding an electron in bonding/anti-bonding MOs
BMO = finding an electron between nuclei is increased w.r.t separated AOs
ABMO = finding an electron between nuclei is decreased (=0) w.r.t. separated AOs
define the overlap integral
interference and the formation of bonding/anti-bonding orbitals is a result of AO overlap. the overlap integral quantifies the overlap
*not given*
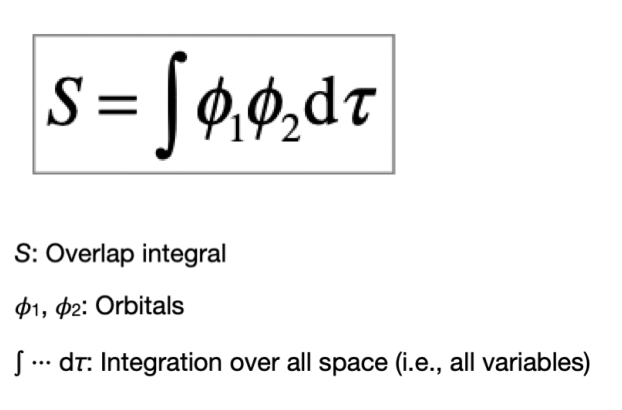
how can symmetry be used to simplify the evaluation of integrals
symmetry tells us which integrals ‘vanish’ (= 0) → which bonding does not occur → non-bonding
if the integral does not vanish (> 0) → calculation to find overlap integral
describe how to construct an MO diagram for a polyatomic
generate SALC and reduce
assign symmetry species (invariant + SALC)
only orbitals of the same symmetry can interact
2 orbital interaction = anti-bonding and bonding
3 orbital interaction = anti-bonding, non-bonding, bonding
closer in e AOs = larger interaction
no. of AOs = no. of MOs
what is a SALC?
symmetry adapted linear combination
describe how to generate a SALC
apply each symmetry operation to each orbital in turn
+1 = no change
0 = moves
-1 = no change, sign flip
sum the characters to give a character table of the reducible representation (pictured)
reduce by either 1) reduction by inspection or 2) reduction formula
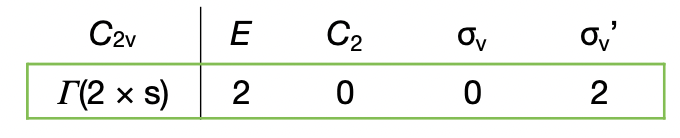
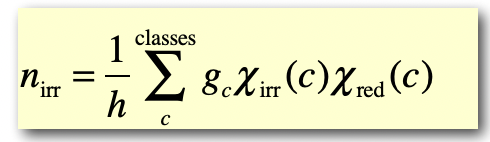
what do the characters in the reduction formula represent?
n(irr) = number of times the irreducible representation occurs in the reducible representation
h = order of the group = total number of symmetry operations =
g(c) = number of symmetry operations in class c
X(irr)(c) = character of class c in the irreducible representation
X(red)(c) = character of class c in the reducible representation
checks:
n(irr) > 0
the dimension (= character under E = number of orbitals combined) = sum of dimensions of the irreducible representations it reduces to
Γ = symmetry species + symmetry species
the ‘orbitals’ form a basis for the ‘symmetry species’ representations in ‘point group’.
describe how to assign symmetry species at an invariant point
s-orbital = ALWAYS totally symmetric symmetry species
other orbitals = read from table
i.e. p(x) = x in table
p(y) = y in table
p(z) = z in table
describe how to determine the shape of SALCs
guess
i.e. add and subtract
projection operators
describe how to determine the shape of SALCs via projection operators
label the orbitals involved i.e. s1, s2, …
select one orbital and for each symmetry operation, note which orbital it is converted into
multiply each “result” by corresponding character in each symmetry species
sum the result to give the linear combination
what do symmetry species e and t mean?
e = accounts for 2 orbitals which are double degenerate
t = accounts for 3 orbitals which are triply degenerate
what does it mean when two features/properties (orbitals) have the same symmetry species?
they are degenerate
symmetry operations must leave the physical properties, including energy, unchanged
what happens when there are >1 (n) degenerate orbitals contributing to a MO?
the resulting MOs (bonding, anti-bonding, non-bonding) are (n) degenerate
describe how to determine the shape of SALCs via projection operators for degenerate orbitals
label the orbitals involved i.e. s1, s2, …
select one orbital and for each symmetry operation, note which orbital it is converted into
multiply each “result” by corresponding character in each symmetry species
sum the result to give the linear combination
repeat 2-4 for the other orbitals we have labelled
sum (MINUS) the result
describe Walsh/correlation diagrams
= shows how the electron structure (MO diagram) varies as a function of a change in molecular structure
y = energy
x = structural coordinate = often bond angle
why do the orbitals between linear and triangular 3H have different energies?
= increasing/decreasing overlap
linear = less overlap possible = more anti-bonding = higher E
triangular = more overlap possible = less anti-bonding = lower E
how is the optimal number of electrons for a molecule found?
fill all the bonding and non-bonding MOs; leave the anti-bonding MOs empty
i.e.
linear H3 = 4e- optimal = H3(-)
triangular H3 = 2e- optimal = H3(+)
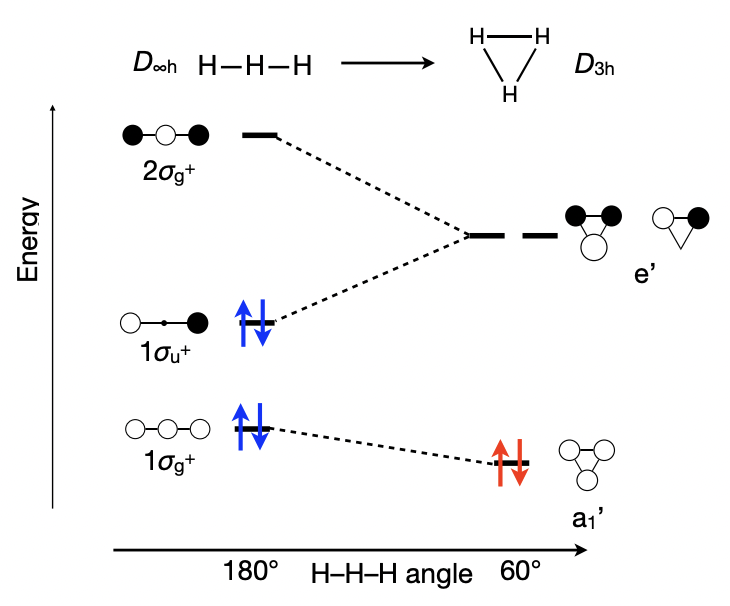
what is Walsh’s rule?
a molecule adopts the structure that best stabilises its HOMO
= hence, changes in the number of electrons can trigger structural change
what type of transition does UV/Vis spectroscopy monitor?
electronic
what is the intensity of electronic transitions defined? *given*
gs = ground state wavefunction
es = excited state wavefunction
μ^ = electric dipole moment operator
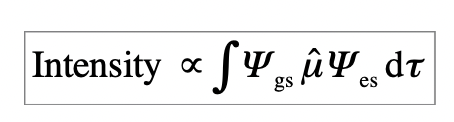
how can group theory be applied to electronic spectroscopy?
= to identify where the integral (of intensity) vanishes (=0) for symmetry reasons
integral vanishes (=0) = transition is dipole-forbidden for symmetry reasons
integral does not vanish (>0) = transition is dipole-allowed
what is the selection rule of a transition being dipole-allowed for symmetry reasons?
a transition is dipole allowed, if and only if, the triple product (of reducible representations) contains the totally symmetric representation
Γgs = symmetry (species) of the ground state
Γes = symmetry (species) of the excited stae
Γ(μ) = symmetry (species) of the dipole-moment operator
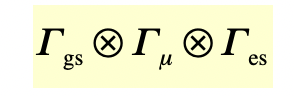
what is the Γ of an electronic state
= direct product of each and every symmetry species of each electron (= AO/MO it occupies)
Γgs = configuration before transition
Γes = configuration after transition
filled/empty orbitals = symmetric = +SS (x) (-SS) = completely symmetric symmetry species
[ SS = symmetry species ]
what is the direct product of 2 symmetry species?
multiply each character in a symmetry species with the equivalent character in the other symmetry species
what is the symmetry of the dipole-moment operator, Γ(μ)?
= same symmetry as the x, y, and z in the point group
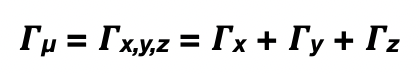
what are the 2 types of spectroscopy measuring bond vibrations?
IR
raman
describe IR spectroscopy
(lower = IR region) energy photon is absorbed by the molecule and excites a vibration
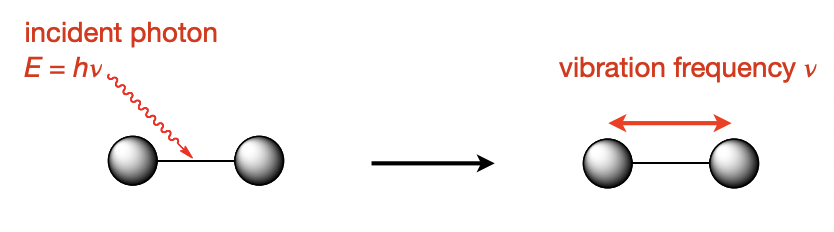
describe raman spectroscopy
(higher = Vis region) energy photon is scattered by the molecule
= the molecule absorbs some energy which triggers vibration
= outgoing photon has lower energy than the incident one
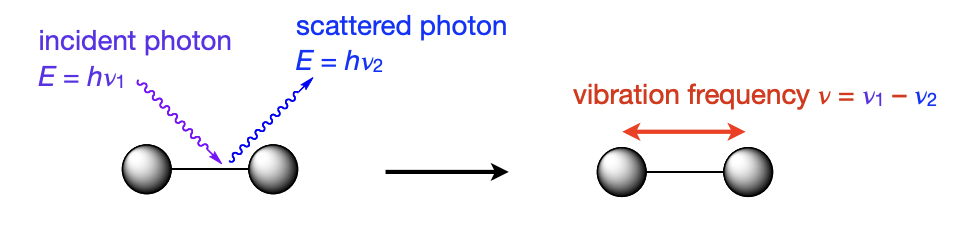
how can group theory be applied to vibrational spectroscopy?
vibrations are displacement of atoms
use Cartesian displacement vectors as a basis to construct representations
describe how to construct a Cartesian displacement reducible representation
determine the point group
place the molecule in the Cartesian coordinate system as defined by the character table
principle rotational axis = z-axis
plane of page = y-axis
apply Cartesian coordinate system to each atom
determine the character of applying each symmetry operation to each Cartesian basis vector (=axis) to each atom
reduce into sum of irreducible representations by reduction formula
what are the 3 types of displacement?
translations
rotations
vibrations
what does the Cartesian displacement reducible representation span?
all 3 types of displacement
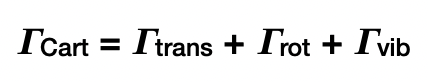
how can the symmetry of translation and rotations be determined?
= in the character table
x, y, z = translations
R(x, y, z) = rotations
how can the symmetry of vibrations be determined?
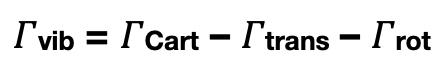
what does a Γ(vib) = 2a1 + b2 mean?
there are two distinct vibrational modes
1 vibrational mode = 1 symmetry species
what is the selection rule of a vibration being IR-active for symmetry reasons?
for any process that involves the absorption of light, the transition is allowed, if and only if, the triple product contains the totally symmetric representation
Γgs = always totally symmetric ~ 1
Γes = Γvib = TREAT EACH VIBRATION SEPARATELY
Γ(μ) = sum of x, y, and z representations in the point group
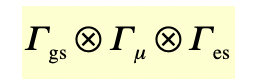
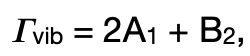
describe the IR-active transition in C2v
a1: IR active
b2: IR active
hence, there are 3 IR-active transitions → 3 IR bands
what is the overall selection rule of IR transitions
a vibration must have the same symmetry (species) as one of the x, y, or z
(i.e. must have a symmetry species matching the dipole moment operator)
what is the selection rule of a vibration being raman active for symmetry reasons?
for any process that involves the absorption of light, the transition is allowed, if and only if, the triple product contains the totally symmetric representation
Γgs = always totally symmetric ~ 1
Γes = Γvib = TREAT EACH VIBRATION SEPARATELY
Γ(a) = symmetry of the polarisability operator = sum of x², y², and z², xy, xz, yz representations in the point group
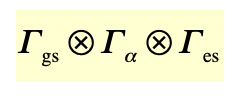
what is the difference in the selection rules for IR and raman activity?
IR activity = triple product w.r.t. symmetry of the dipole-moment operator
Raman activity = triple product w.r.t. symmetry of the polarisability operator
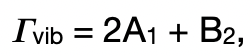
describe the raman-active transition in C2v
a1: raman active
b2: raman active
hence, there are 3 raman active transitions → 3 raman bands
what is the overall selection rule of raman transitions
a vibration must have the same symmetry (species) as one of the x², y², z², xy, xz, zy
(i.e. must have a symmetry species matching the polarisability operator)