Organic Chemistry - Functional Groups and General Vocab
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Alkane
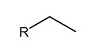
Alkene
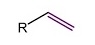
Alkyne

Aromatic (Arene)
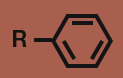
Ether
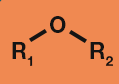
Alcohol

Amine

Carboxylic Acid
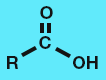
Aldehyde
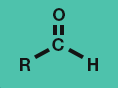
Ketone
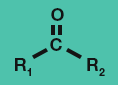
Ester
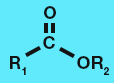
Amide
Anti
Opposite
Guache
Close
constitutional isomer
compounds with the same molecular formula but a different connectivity of their atoms
stereoisomers
compounds that are different due to spatial arrangement of atoms, not bond rotation
enantiomer
non-superimposable mirror images, all stereo-centers
diastereomer
stereoisomers are not mirror images
Lewis Acid
e- pair acceptor
Lewis Base
e- pair donor
Bronsted Acid
H+ donor
Bronsted Base
H+ acceptor
Strong Acid
Small pka, weak conjugate base, conjugate base is a stable ion
Weak Acid
larger pKa, strong conjugate base, conjugate base not stable ion
Position of equilibrium favors reaction of
weaker acid/weaker base pair
meso compound
contains chiral centers but is overall a-chiral due to internal plane of symmetry
HClO4, HCl, HBr, HI, HNO3, H2SO4
Strong Acids
LiOH, NaOH, KOH, Ca(OH)2, Sr(OH)2, Ba(OH)2
Strong Bases
Thiol
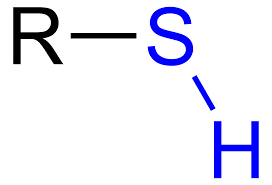
prop-
3
but-
4