Molecular Biology of Cancer 2: What are the cancer hallmarks?
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What is the six original hallmarks of cancer?
Proposed as a set of 6 functional capabilities that are crucial for their ability of cells to form malignant tumours, essentially rules governing the transformation of normal cells into malignant cancer
What are the six original hallmarks dictating malignant growth? VERY IMPORTANT.
1. Self sufficiency in growth signals
2. Insensitivity to 'anti-growth' signals
3. Evasion of apoptosis
4. Limitless replicative potential
5. Sustained angiogenesis
6. Tissue invasion and metastasis
What are the proposed four ‘emerging’ hallmarks of cancer?
7. Avoiding immune destruction
8. Tumour promoting inflammation
9. Genome instability and mutation
10. Deregulating cellular energetics
What does a normal cell require to grow?
What do cancer cells do that allows continual growth?
Normal cells require positive growth signal (Which is a mitogen), or else it will remain in G1, no growth factors = no cell division
Cancer cells can grow with or without the presence of growth factors
Cancer Hallmark 1
What three ways can cancer cells bypass positive growth signals?
Also note some examples
Cancer cells can alter the levels of growth signals by synthesising their own growth signals
Autocrine signaling
Cancer cells can synthesise more growth receptors or modify growth receptor into a 'on' constant state
E.g. HER2 receptors on breast cancer cells, synthesizes more → results in more growth + responsiveness to hormones → cells grow faster
Is also a proto oncogene
Cancer cells can modify downstream signalling pathways- signal to divide/proliferate is 'on' constantly
e.g. Oncoprotein Ras is an proto-oncogene, key downstream component in growth factor signaling pathways, when mutated Ras oncogene is permanently activated, mutant Ras is permanently switched on
Bypasses the need for growth factor or growth receptor
Normally needs a growth factor for signaling pathway to be activated
Cancer Hallmark 2
Reminder, what three growth suppressor or tumour suppressor pathways inhibit cell growth? What happens if they become mutated?
In response to DNA damage- p53 will arrested in G0, prevent cell cycle progression
Many cancer cells (>90% have mutated p53- looses the wildtype p53 function
Mutant p53 cannot trigger cell cycle arrest or apotosis
The tumour suppressor pRb can function as a negative regulator of the cell cycle; when no cyclin/CDK complex is present the cell is halted in G1
However, if pRb is mutated it remains in the phosphorylated status and the cell will proliferate (no requirement for cyclin/CK)
Cancer Hallmark 4
How do cancer cells achieve immortality?
• Most normal cells can only divide a finite number of times before entering the non dividing state of senescence
• Number of cell divisions is controlled by telomeres
• Majority of cells however, can maintain telomere lengths throughout cell division and hence the signal to enter to senescence is bypassed and these cells can divide indefinitely and termed immortal cells
• Immortality is achieved by expression of an enzyme called TELOMERASE that can synthesise telomere DNA back onto the end of the chromosome, so whilst the end replication looses DNA, telomerase resynthesizes telomere DNA
Cancer Hallmark 3
How do cancer cells evade apoptosis?
Tumour suppressor p53
• p53 is a key trigger of apoptosis in response to DNA damage
Function is abrogated in over many tumours, ability to trigger apoptosis is compromised
BCL-2
• Bcl-2 is a proto-oncogene that is anti -apoptotic
Overexpression (oncogene activation) results in loss of apoptotic function
What is angiogenesis?
• Angiogenesis is the growth of new blood vessels from pre-existing ones
It's a normal physiological process e.g involved in embryogenesis, wound healing and expulsion of uterine lining during menstruation
Cancer Hallmark 5
Why is tumour growth limited by blood supply?
The ability of tumour to absorb nutrients is proportional to its surface area; 0.2mm is the distance that oxygen can effectively diffuse through living tissues
As tumour grows the outer cells proliferate as they have access to they blood supply but the inner cells become hypoxic and tumour 'cores' furthest from the blood supply can become necrotic
In order for the tumour to be able to divide it needs an additional blood supply which is provided by angiogenesis; synthesis of new blood vessels from existing vasculature
Hypoxia induces quiescence (pushes cell out of cell cycle in GO)
For a tumour to grow it needs to be able to synthesis more blood vessels via angiogenesis
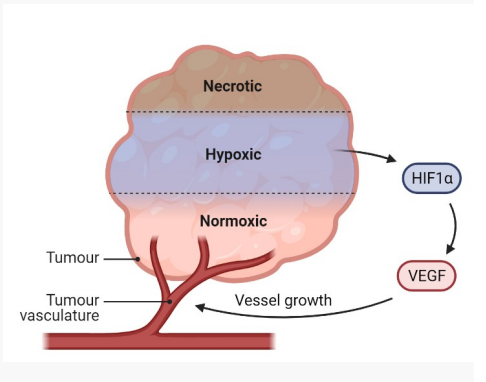
How do tumour cells stimulate angiogenesis?
Hypoxia induces the expression of Hypoxia Inducible Factor-1 (HIF- 1)
HIF-1 triggers activation of Vascular Endothelial Growth Factor (VEGF)
VEGF (growth factor) interacts with receptors on endothelial cells (cells line blood vessels) and this triggers a signaling cascade
Signalling cascade triggers the proliferation and migration of endothelial cells to form new blood vessels
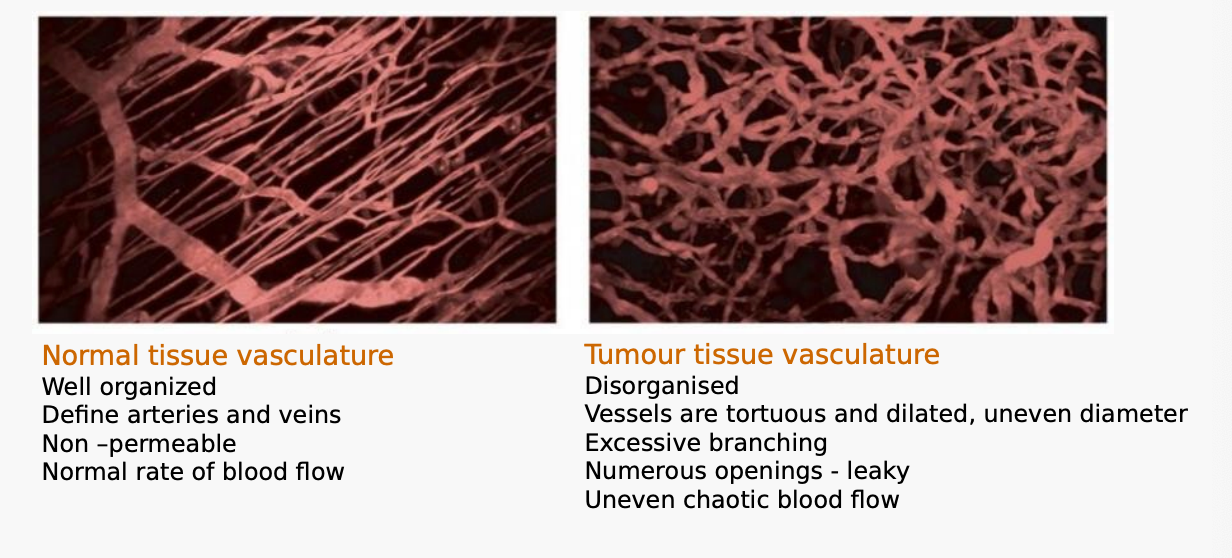
Compare the appearance of normal tumour vasculature with that of tumour tissue vasculature.
What is tumour cell invasion?
Ability of tumour cells to detach from primary tumour and invade surrounding tissues
What is metastasis?
The spread of cancer cells from the point of origin to another part in the body via blood, lymphatics most commonly
What is a primary tumour?
What is a secondary tumour?
Tumour cells at the original site
Cancer cells found at metastatic sites
Is there a difference between the cell type in a metastatic tumour versus a primary tumour?
NO, cell type in primary = cell type in metastatic
Metastatic causes 90% of cancer deaths
Cancer Hallmark 6
What are the 5 steps in the Invasion and Metastasis Cascade?
Metastatic cancer cell detaches from the primary tumour, migrates and invades the basement membrane (Proteolysis)
Changes in cell adhesion allows the cells to break away
Intravasation of metastatic cancer cell
Involves invasion of the ECM (Proteolysis) in order to create a path to vessels
Mechanism poorly understood
Metastatic cancer cells travel in lymphatic or blood vessels
Extravasation of metastatic cancer cells
Metastatic cancer cells grow at a site other than the primary site
Describe the normal epithelial cell architecture.
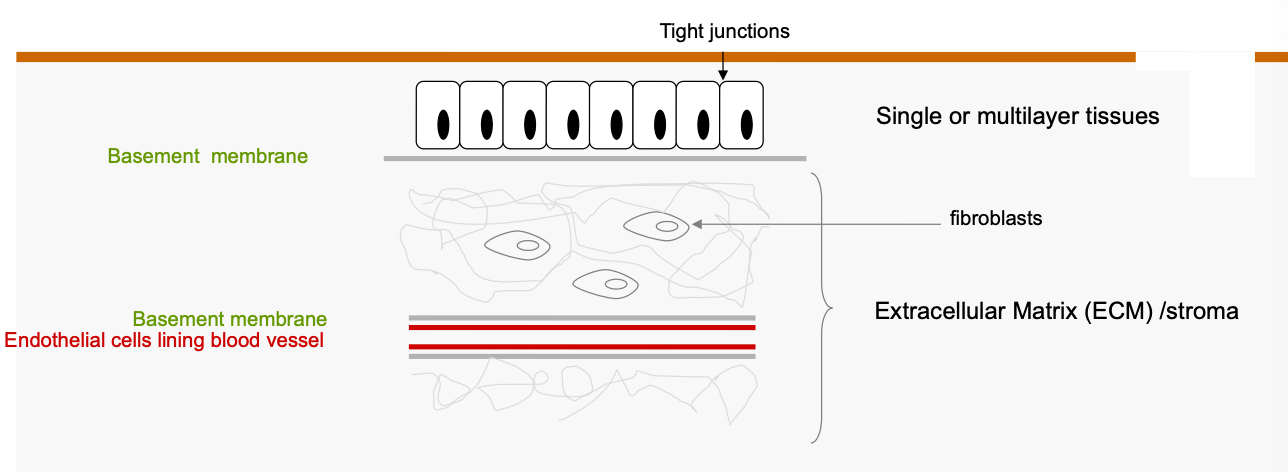
To becoming invasive, cancer cells must breach the ________ ______.
basement membrane
Describe how tumour cells are transported through blood and the danger they may face.
Blood is a hostile environment for cancer cells
Hydrodynamic forces can shear cells
May face Immune Attack
Cell can die by Anoikis
Form of cell death caused by detachment from substrate
Evidence to suggest that groups of cancer cells bind platelets for transport → Principle mechanism of transportation, platelets provide a shelter
Metastatic Ineffiency - basically it is not an efficient process because they cannot survive the circulatory pathway
E.g. 106 cells per day can leave a primary mouse tumour (~10' cells) and enter the circulation but most do not form clinically relevant tumours
What are the two models of metastasis believed to explain why some cancers metastasize and others do not?
• Multi step model
Step wise accumulation of genetic and epigenetic (DNA methylation and histone modification) alternations leading to a more aggressive phenotype
Sub populations of cells with metastatic potential
• Cancer Stem cells
Are essentially "Cancer initiating cells" that may stay latent in the primary tumour and escape to metastasis when induced by external factors, such as: hypoxia
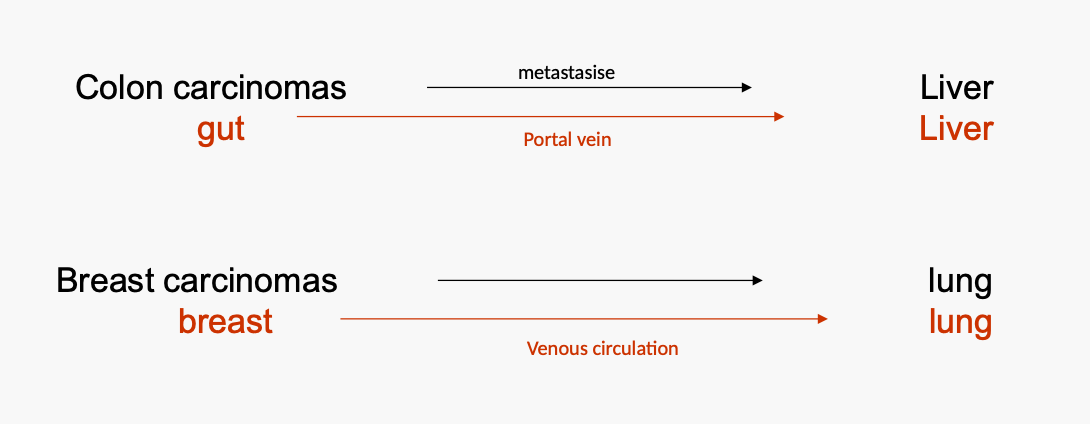
What is the Seed and Soil Theory or reason for organ selectivity regarding cancer?
"Seed and soil" theory- Paget 1889
Cancer cells are scattered randomly through out the body but only those that land is a hospitable environment will survive
'Specialised microenvironment'
But......Contralateral metastases are rare
In only 2% of breast cancers patients are metastases detected in the contralateral breast
Kidney tumours metastasise infrequently to contraleral kidneys
What is the mechanistic theory or reason for organ selectivity regarding cancer?
Mechanistic theory:
Determined by the pattern of blood flow.
Consequence of the anatomical location of a primary tumor