Bond energy calculations.
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
What are exothermic energy changes shown as
Energy is transferred to surroundings, therefore the energy changes are shown as negative.
What are endothermic energy changes shown as.
Endo energy changes shown as positive as they have gained energy from surroundings.
Explain concept of bond energies.
When we break chemical bond, it requires energy (endo)
Making bonds releases energy (exo)
Every chemical bond has an energy value
This tells us the energy required to break that bond
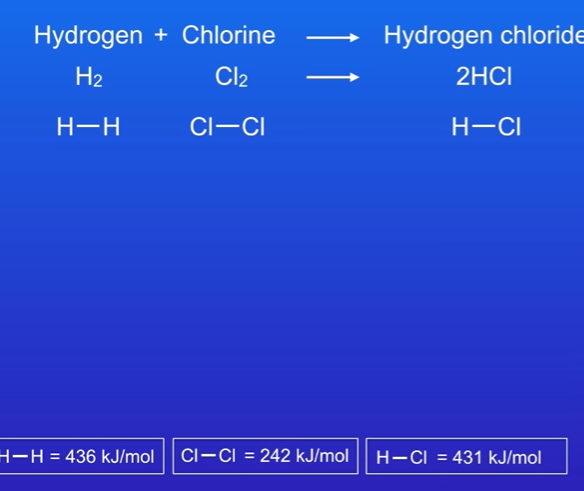
Calculate energy change for hydrogen + chlorine → Hydrogen chloride.
Balance first.,
On left side , we are breaking chemical bonds, so these bonds are being broken (H-H, Cl-Cl) .
On the right hand side, we are making chemical bonds, so this bond is being made.
Put in the energy values, breaking bond requires energy , so +436 is value for breaking H-H bond and +242 for breaking Cl-Cl bond. Add these values, and you get +678.
On right hand side, bond is being made and released 431 kj/mol. This must be multiplied by 2 as we are making 2 molecules of hydrogen chloride, si 431 × 2 = 862. Making bond releases energy , so its -862
Work out final energy change by taking away -862 from +678, which is -184 KJ, shows reaction is exothermic.
