Crude oil
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Crude oil is
is a finite resource that is found in the Earth’s crust. It is the remains of organisms that lived and died millions of years ago - mainly plankton which was buried in mud.
Crude oil is an important source of:
fuels such as petrol, diesel, kerosene, heavy fuel oil and liquefied petroleum gases
feedstock for the petrochemical industry
What is a feedstock
A feedstock is a raw material used to provide reactants for an industrial reaction
List other useful substances made from compounds found in crude oil:
Solvents
Lubricants
detergents
Fractional Distilation is used for:
Fractional distillation is used to separate crude oil into simpler, more useful mixtures. This method can be used because different hydrocarbons have different boiling points.
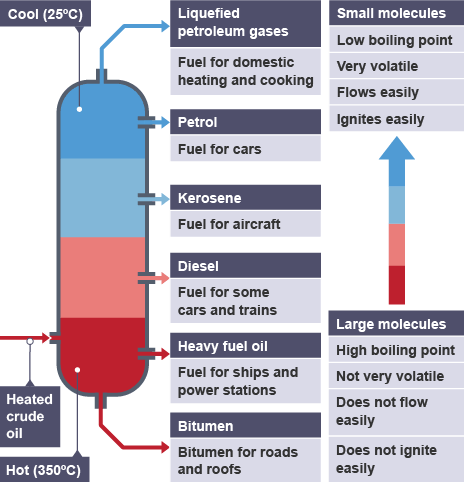
properties of hydrocarbons
Long chain hydrocarbons have a high boiling point
Short chain hydrocarbons have low boiling points
Long chain hydrocarbons have high viscocity
Short chain hydrocarbons have lower viscosity
What happens during the disstilation of crude oil:
Heated crude oil enters a tall fractionating column which is hot at the bottom and cooler towards the top, vapours condense, liquids are led out of the column at different heights, Small hydrocarbon molecules have weak intermolecular forces so they have low boiling points, they do not condense but leave the column as gases. Long chain hydrocarbon molecules have stronger intermolecular forces, so they have high boiling points, They leve the column as hot liquid bitumen.
Properties of fractions - Each crude oil fraction contains a mixture of hydrocarbons. The hydrocarbons in a fraction are mostly hydrocarbons called alkanes. They have similar (but not identical):
numbers of hydrogen and carbon atoms in their molecules
boiling points
ease of ignition
viscosity
kComplete Combustion
Complete combustion of a hydrocarbon fuel happens when there is a good supply of air. Carbon and hydrogen atoms in the fuel react with oxygen in an exothermic reaction:
carbon dioxide and water are produced
the maximum amount of energy is given out
Incomplete combustion
Incomplete combustion happens when the supply of air or oxygen is poor. Water is still produced, but carbon monoxide and carbon are produced. Less energy is released than during complete combustion.
What is cracking
Cracking is a reaction in which larger saturated hydrocarbon molecules are broken down into smaller, more useful hydrocarbon molecules,