Kinetic Molecular Theory SMDT
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
The particles of a liquid have (more, less)____________ mobility than the particles of a gas do.
less
The fact that the particles of a liquid are very close together accounts for a liquid's _____ volume and incompressibility.
definite
The process by which liquids change to gases quickly at one temperature is called
vaporization
The process by which liquids change to a gas gradually over a range of temperatures is called
evaporation
The process of ____________ occurs when the energy of the particles decrease to the point at which attractive forces pull the particles into fixed positions.
freezing
Solids still have measurable temperatures because their particles still _________about fixed points.
vibrate
The ____________ point of a solid is the temperature at which the particles of a solid gain enough energy to overcome the attractive forces holding them in fixed positions and they are free to move around and flow.
melting
Solids are incompressible because their particles are __________.
close together
The kinetic-molecular theory of matter states that particles of all matter are _____.
always in motion
When liquids change into solids, they are said to _____.
freeze
________are more ordered than________.
Liquids, gases
________are less ordered than________.
Liquids, solids
_____and_____are incompressible and have definite volumes because their particles are close together.
Solids, liquids
_____are compressible because their particles are far apart.
Gases
Because liquid and gas particles have enough energy to overcome some of the forces between them, the particles have freedom of motion to _____ with no definite _____.
flow, shape
Because the intermolecular forces in a solid hold the particles in relatively fixed positions, solids have a _____ _____.
definite shape
A gas changing to a solid is called _____.
deposition
A solid changing to a gas is called_____.
sublimation
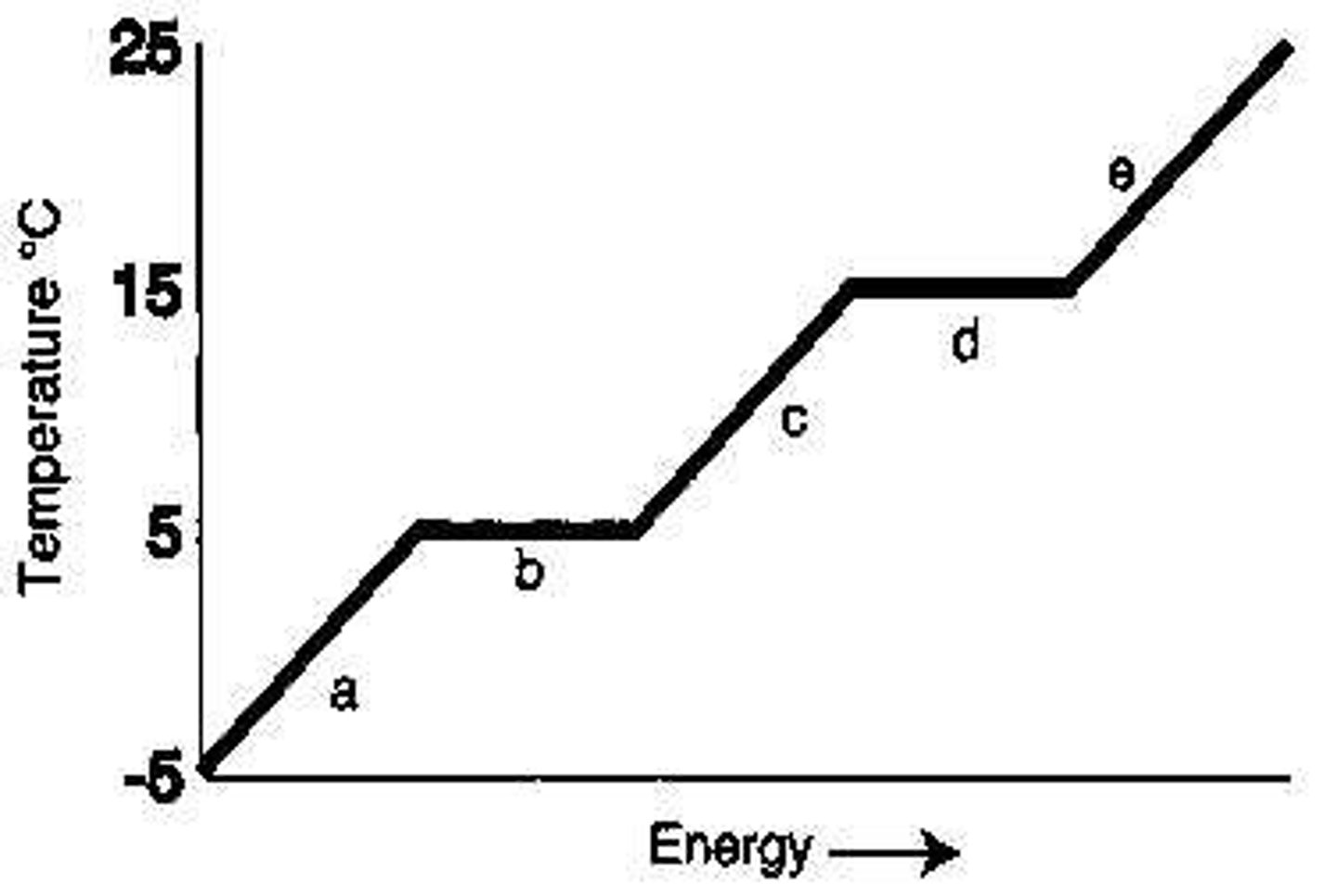
From the diagram, the melting point of the substance is _____°C.
5
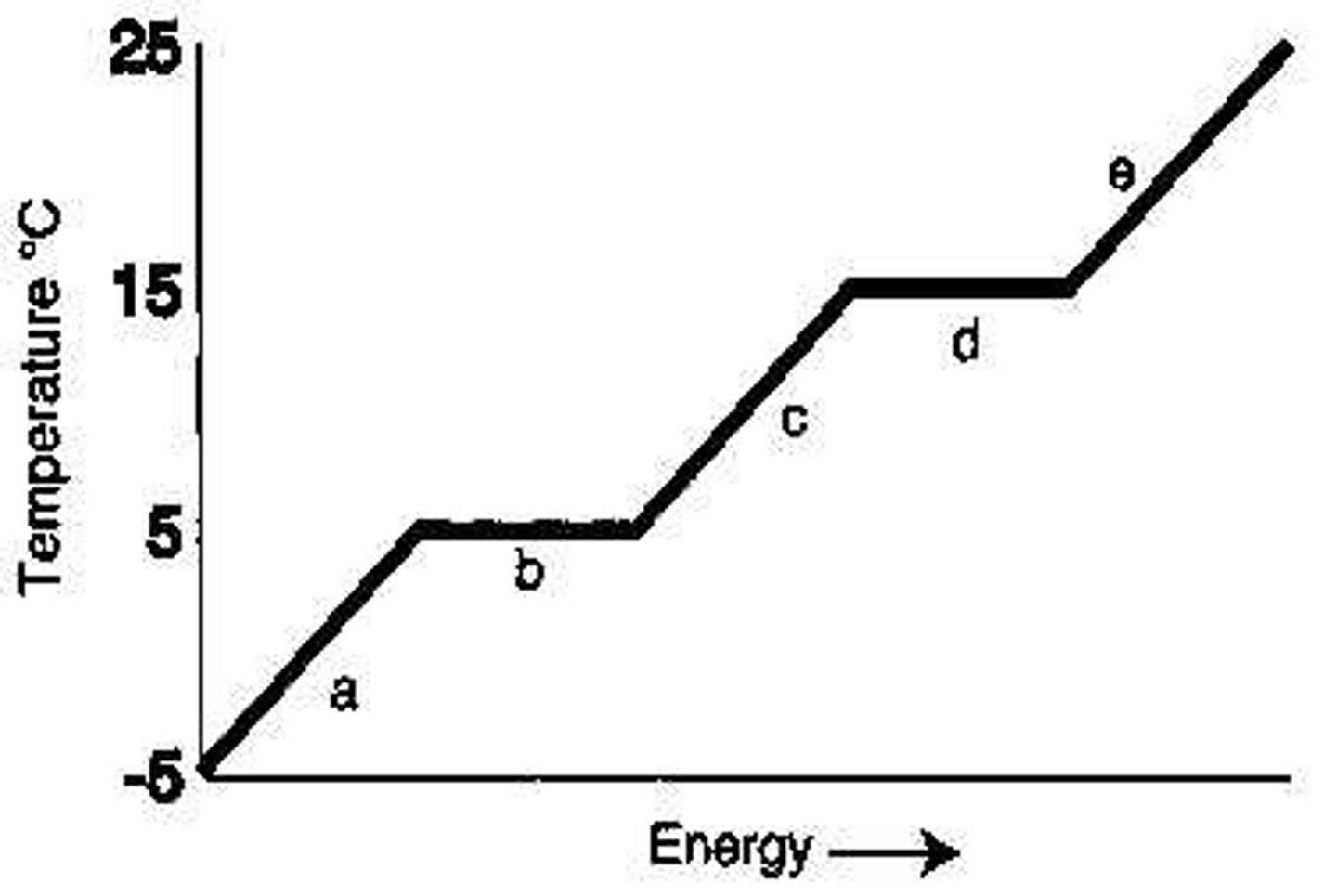
From the diagram, the boiling point of the substance is _____°C.
15
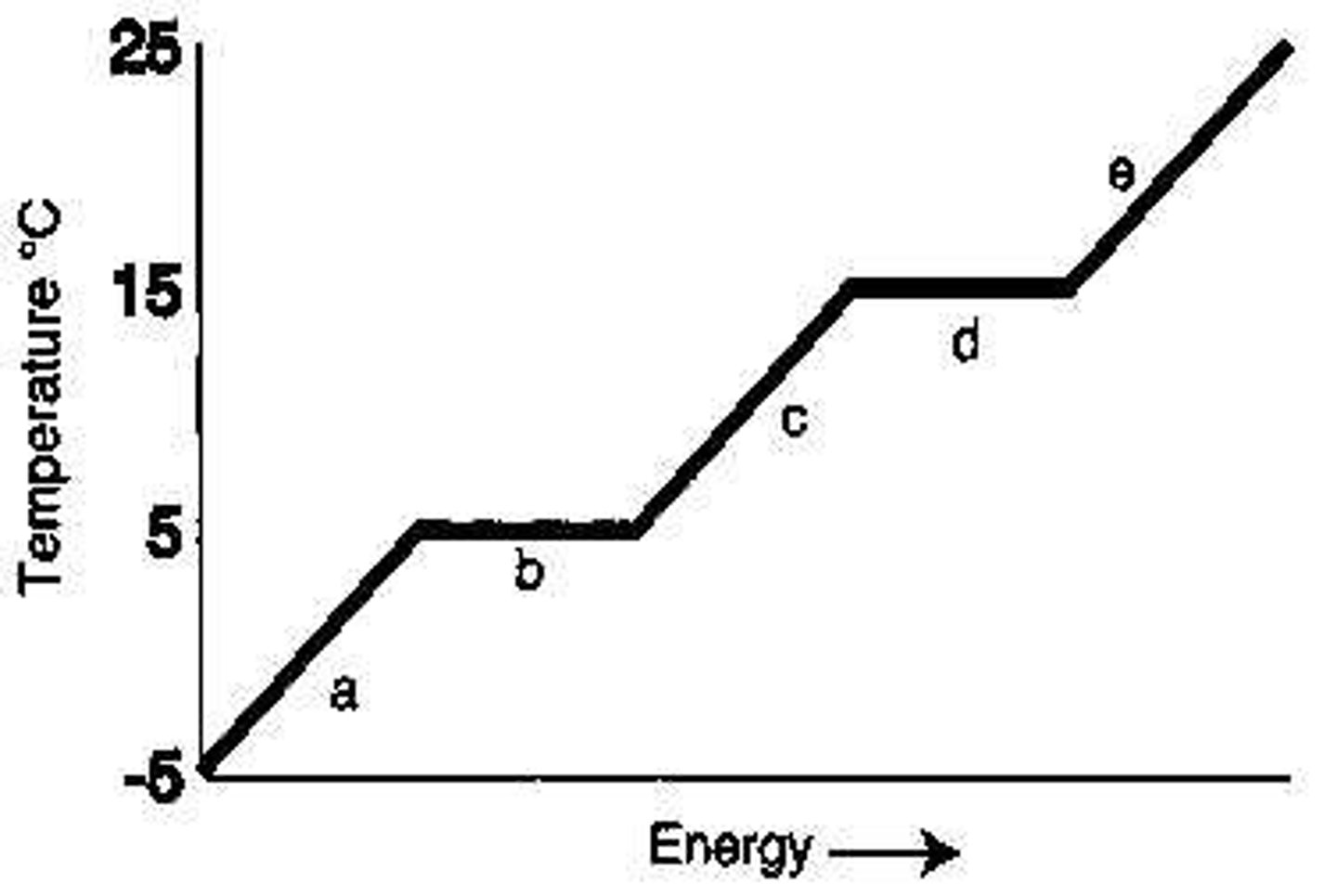
Which letter represents heating of the solid?
A
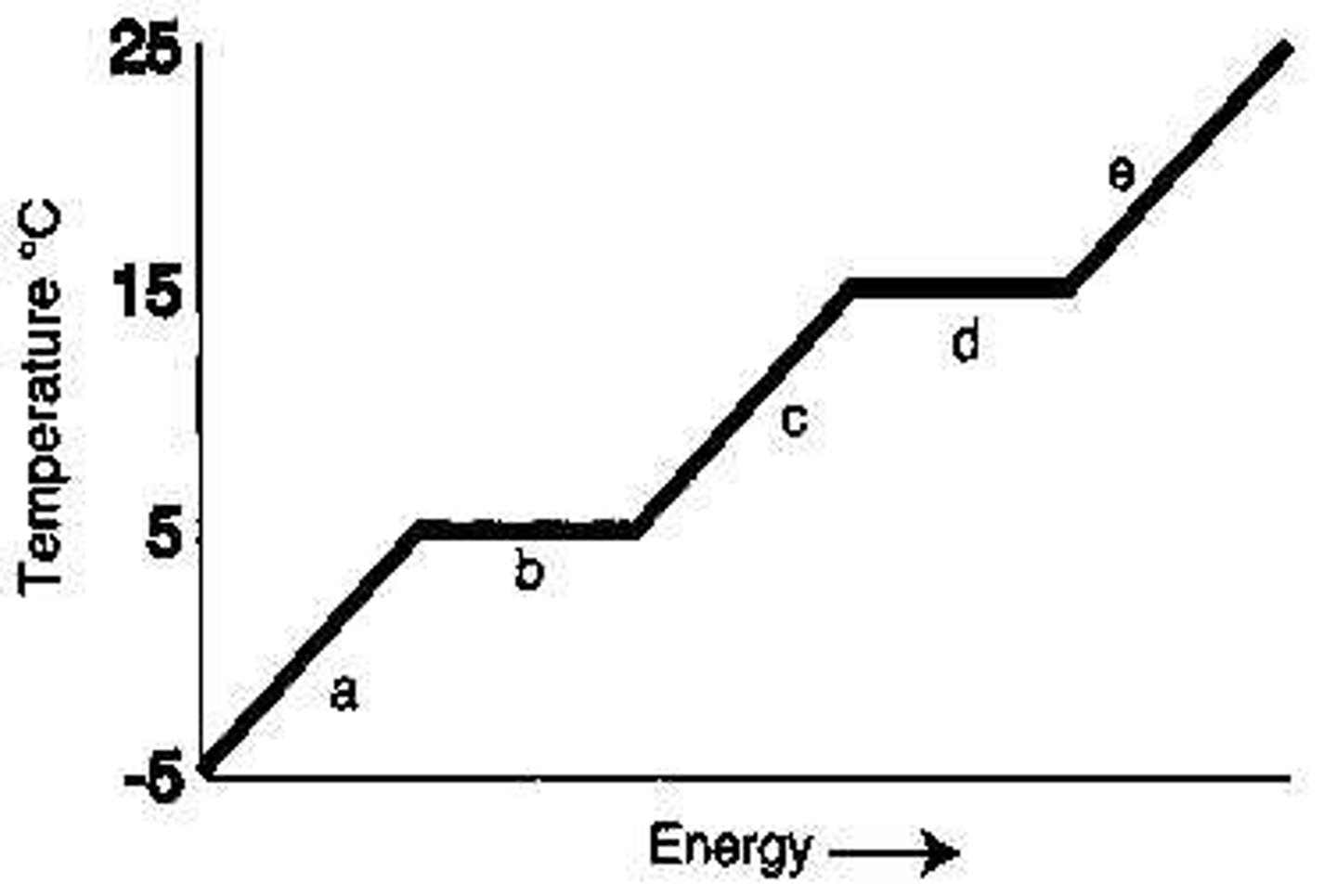
Which letter represents heating of the vapor?
E
A measurement of average kinetic energy of the particles in a substance is _____.
temperature
As the temperature on a material increases, the particles move_____.
faster
When a gas changes to a liquid, it is called_____.
condensation