Unit 1- States of Matter
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
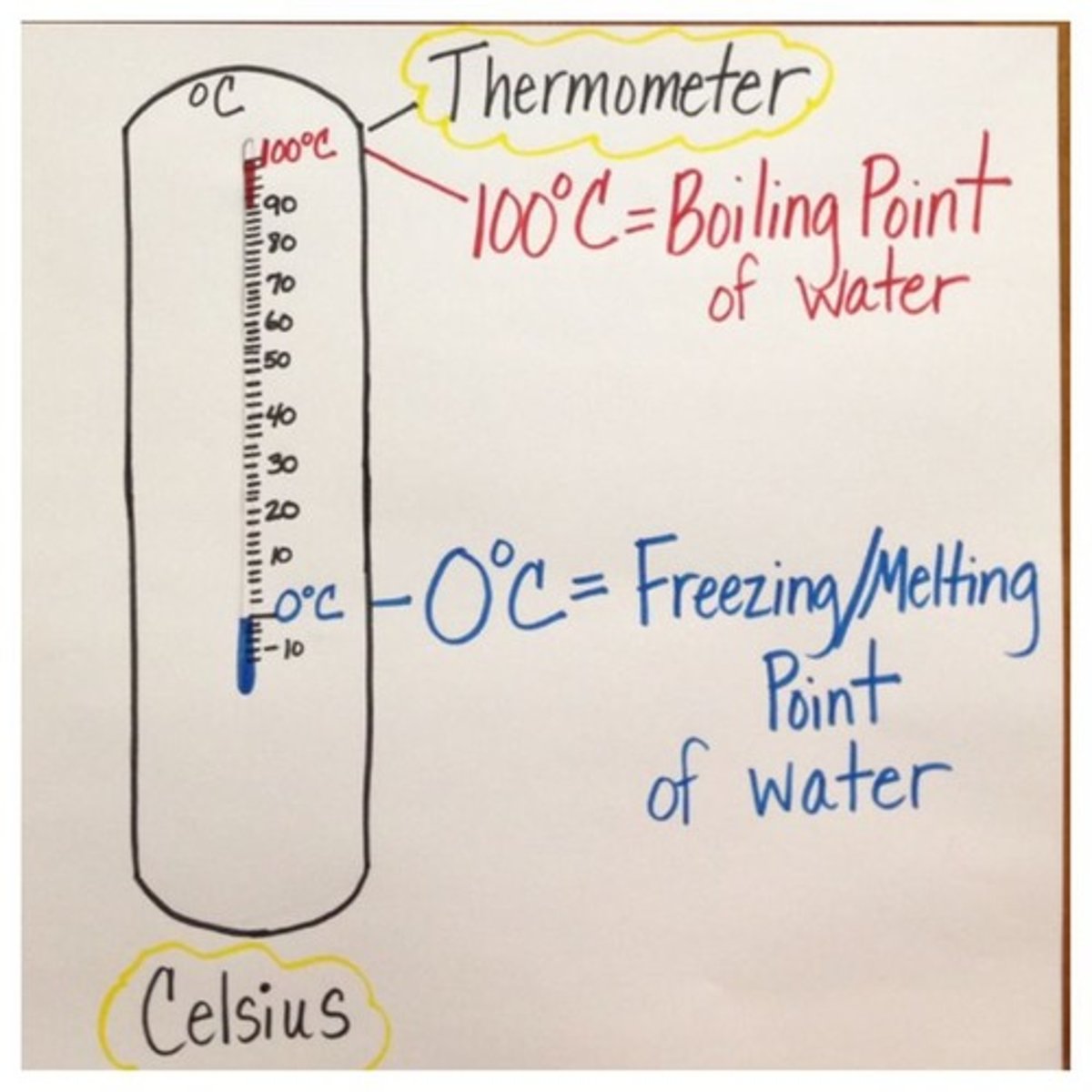
Boiling Point
The temperature at which a liquid changes to a gas

melting point
The temperature at which a solid becomes a liquid

Boiling
vaporization that occurs throughout a liquid

Condensation
The change of state from a gas to a liquid

Deposition
The change of a substance from a gas directly to a solid

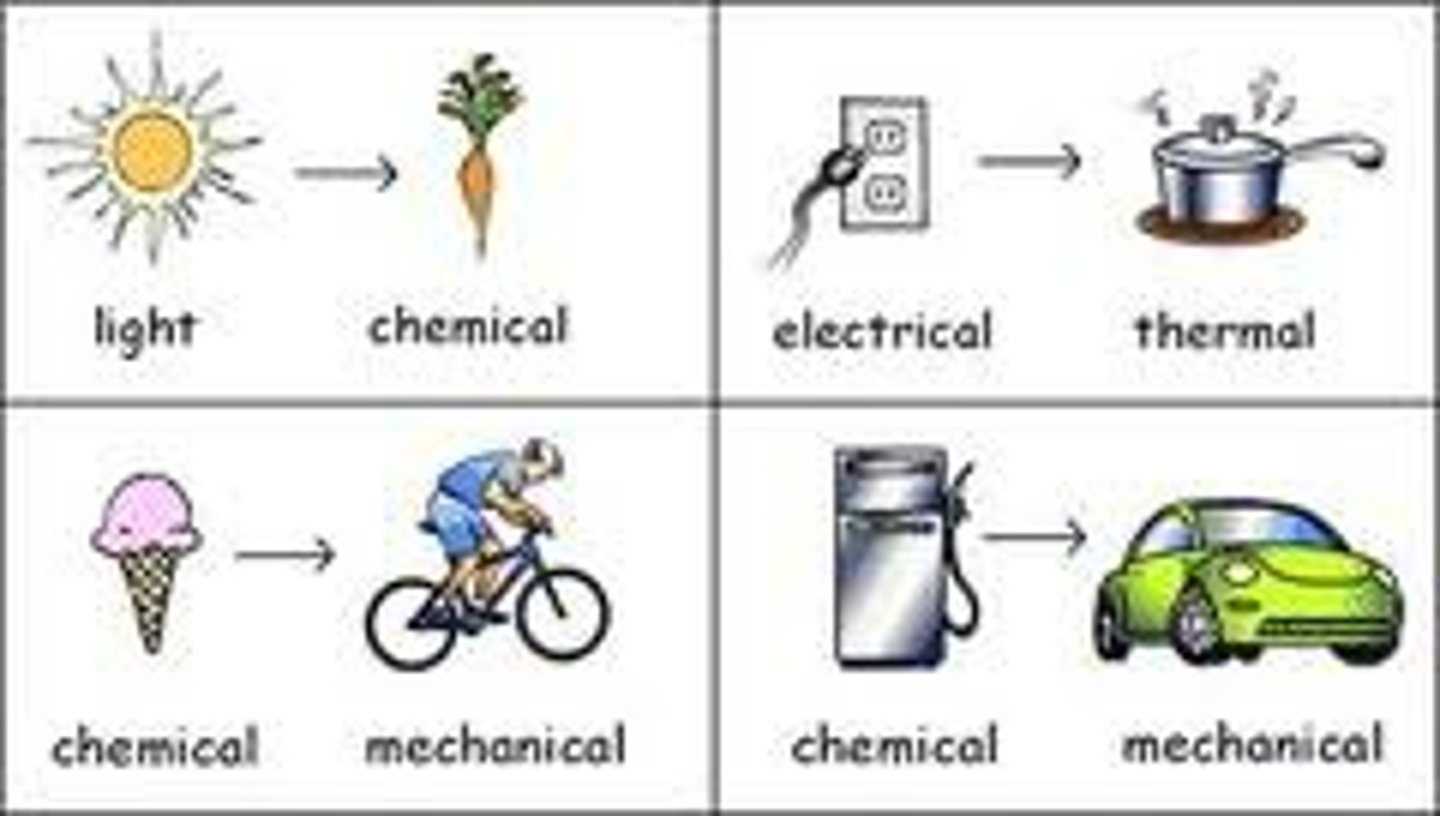
Energy
The ability to do work or cause change
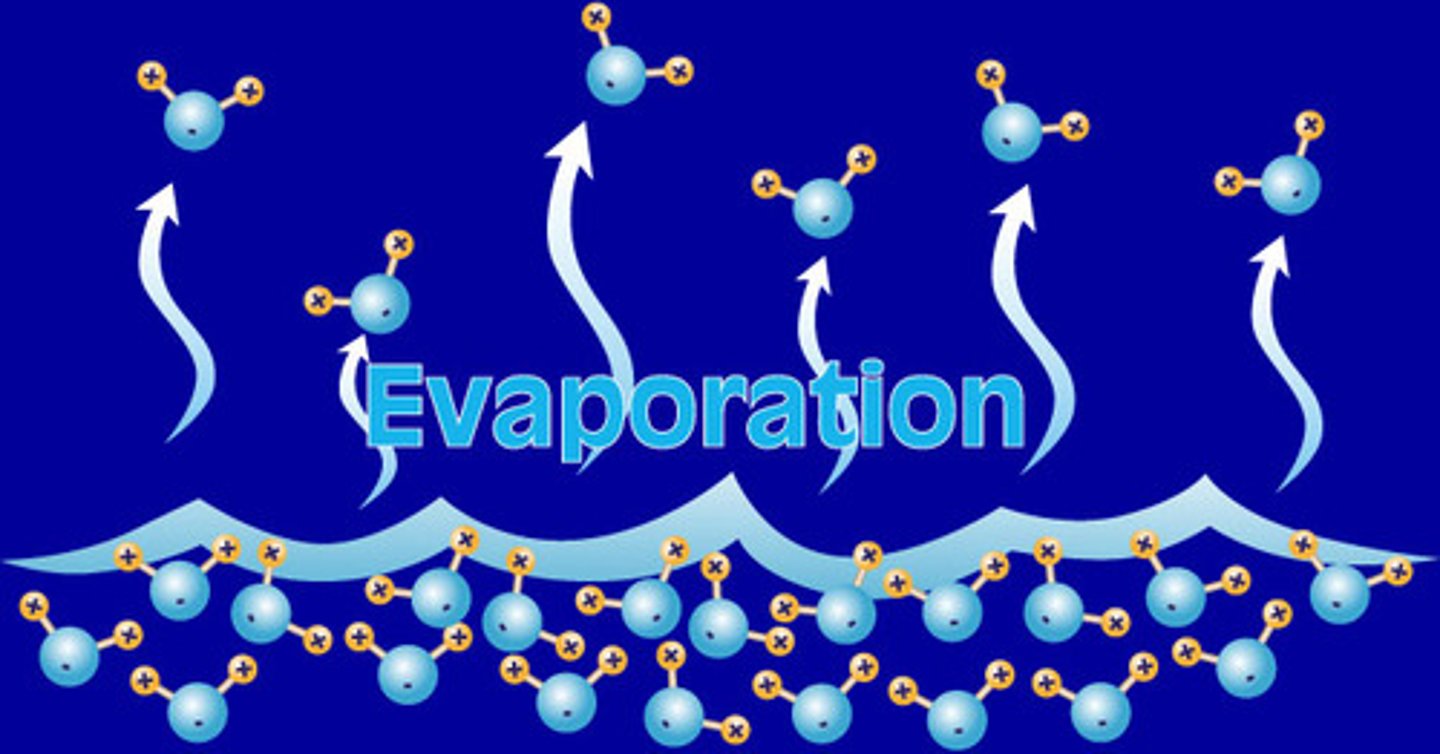
Evaporation
The change of a substance from a liquid to a gas
Fluid
A substance that flows

Freezing
The change of state from a liquid to a solid

Gas
A state of matter with no definite shape or volume
liquid
A state of matter that has no definite shape but has a definite volume.

Kinetic Energy
energy of motion

Kinetic Theory of Matter
states that all of the particles that make up matter are constantly in motion
Mass
The amount of matter in a substance

Melting
The change in state from a solid to a liquid

Melting Point
The temperature at which a solid becomes a liquid
Molecule
group of two or more atoms that form the smallest identifiable unit of a pure substance
Molecular Motion
The motion of the particles found in matter
Phase Change
a change from one state (solid or liquid or gas) to another without a change in chemical composition
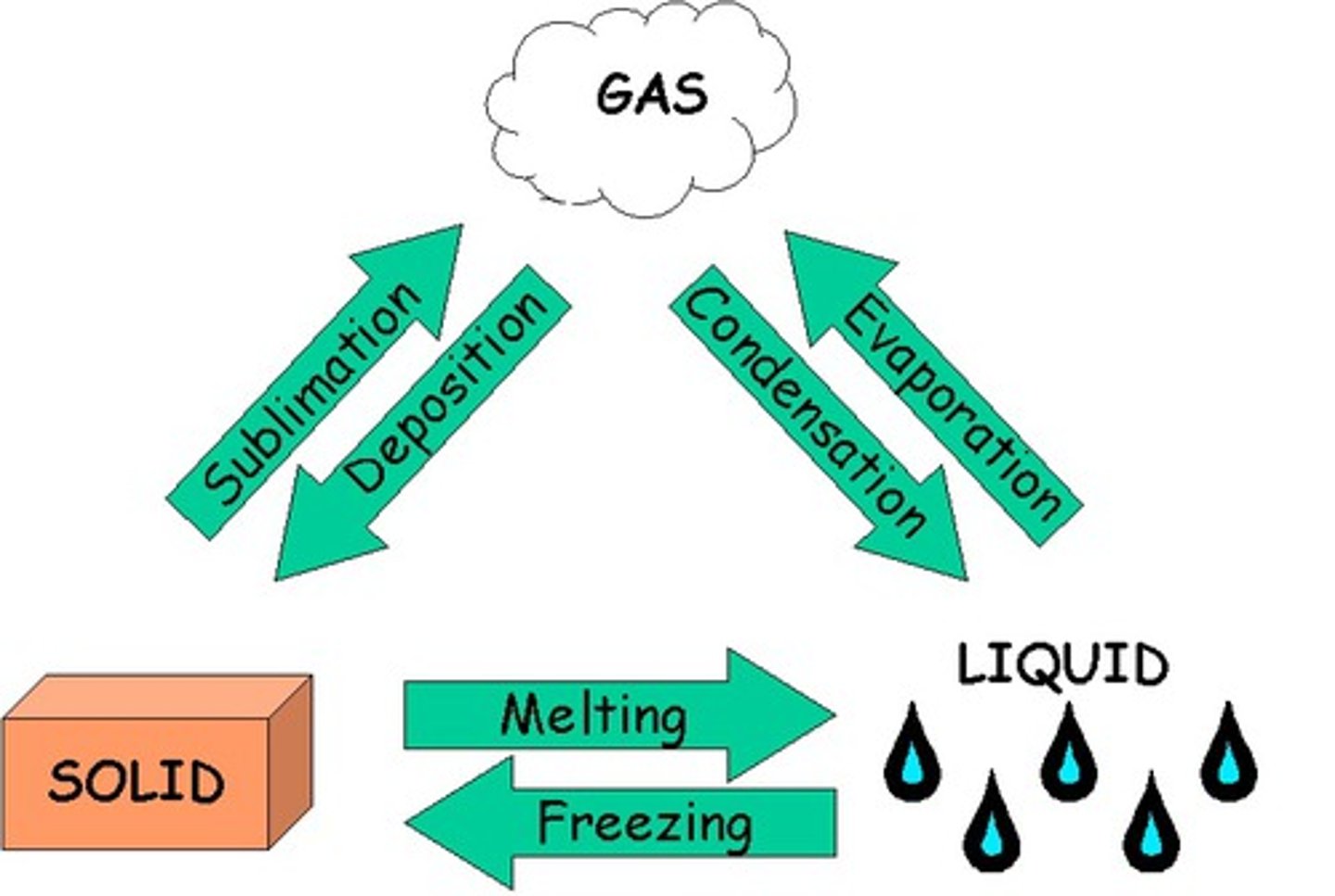
Plasma
gases that have been so energized that their atoms have been stripped of some or all electrons.

Pressure
the amount of force exerted per unit area of a surface

Solid
A form of matter that has a definite shape and volume

Sublimation
A change directly from the solid to the gaseous state without becoming liquid

Thermal Energy
The total energy of motion in the particles of a substance
Vaporization
a physical process that results in the phase transition of a substance from a liquid to a gas.
Volume
The amount of space an object takes up
