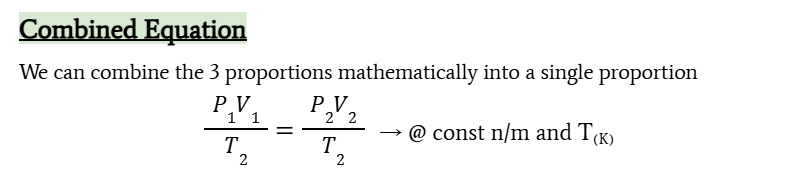12.1 Combined Gas law
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
28/5/25
Last updated 10:41 PM on 5/28/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
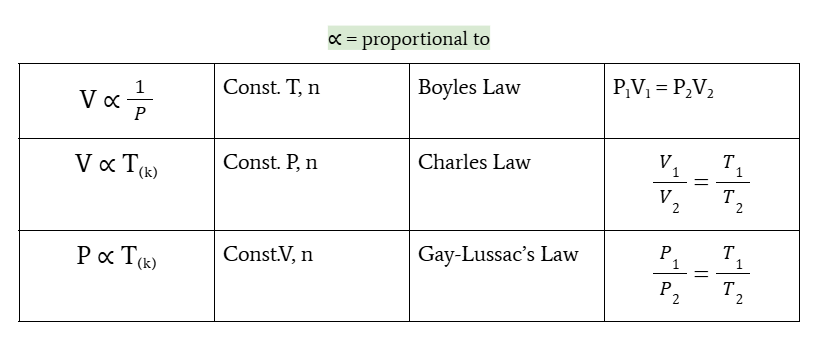
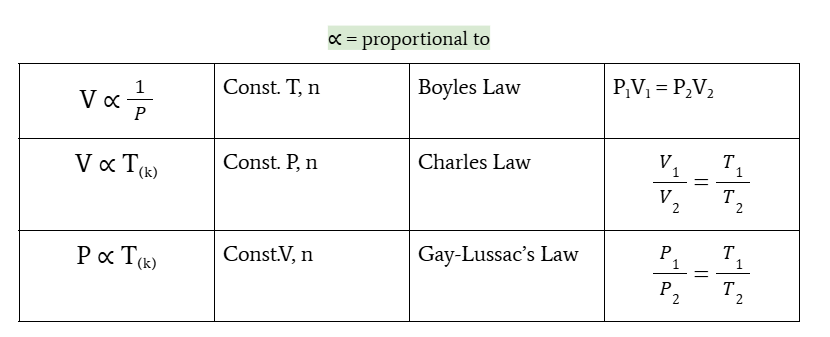
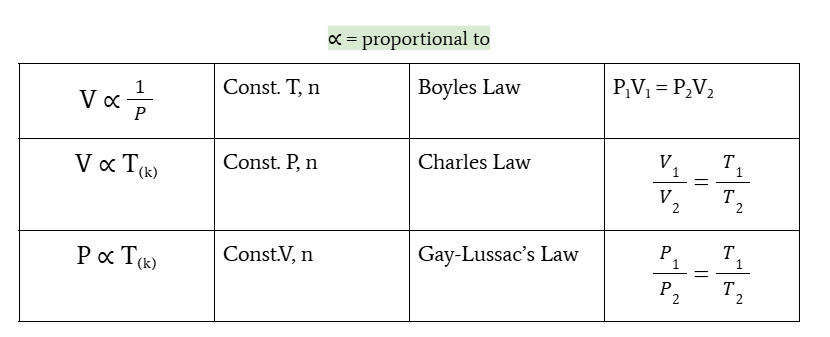
relationship of volume and pressure
volume is inversely proportional to pressure at constant T and n
Boyle’s law
P1V1 = P2V2
2
New cards
relationship of volume and temperature
volume is directly proportional to temperature at constant P and n
charles’s law
V1/V2 = T1/T2
3
New cards
relationship between pressure and temperature
pressure is directly proportional to temperature at constant V and n
gay-lussac’s law
P1/P2 = T1/T2
4
New cards
combined gas law
P1V1/T1 = P2V2/T2 at constant n/m and T in Kelvin