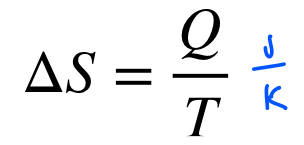Thermal Energy & Thermodynamics
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
converting celsius to kelvin
TC=TK-273.15
coverting farenheit to celsius
TF= 9/5TC+32
thermal expansion
change in size or volume of a given mass with a temperature change
a= coefficient of linear expansion (units: 1/oK or oC)
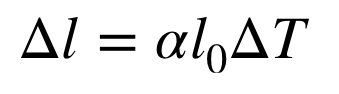
average kinetic energy of molecules within a gas
Kavg=1/2mv2
Kavg=3/2 x KBT
Kavg=3R/2N x T
Boltzman’s constant (KB)
1.38 × 10-23J/K
what are the 3 methods of heat transfer?
conduction
convection
radiation
conduction
heat transfer vis direct contact
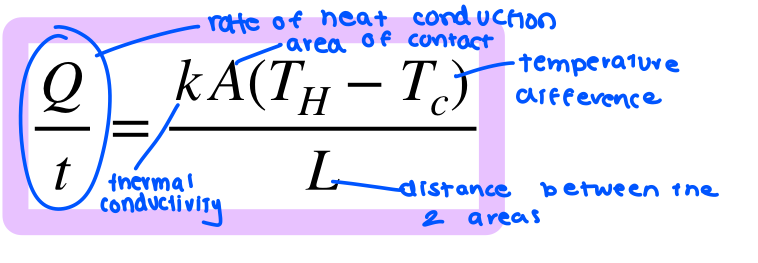
convection
heat transfer that occurs within a fluid (liquid or gas) due to a difference is density between regions
radiation
heat transfer without any physical contact via electromagnetic waves
ex: warming of the Earth by the Sun
what is the 0th law of thermodynamics?
if two systems are in equilibrium with a 3rd system, then they are in equilibrium with each other
what is the first law of thermodynamics?
change in internal energy of the system is equal to the work done on the system or by the system plus or minus the heat that flows in or out of the system
W>0: work is done ON the system
W<0: work is done BY the system
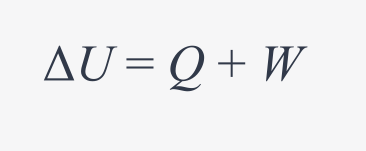
1st law as it relates to the 4 thermodynamic processes (draw chart!)
*NOTE: these are all irreversible processes as the final and initial states differ from each other
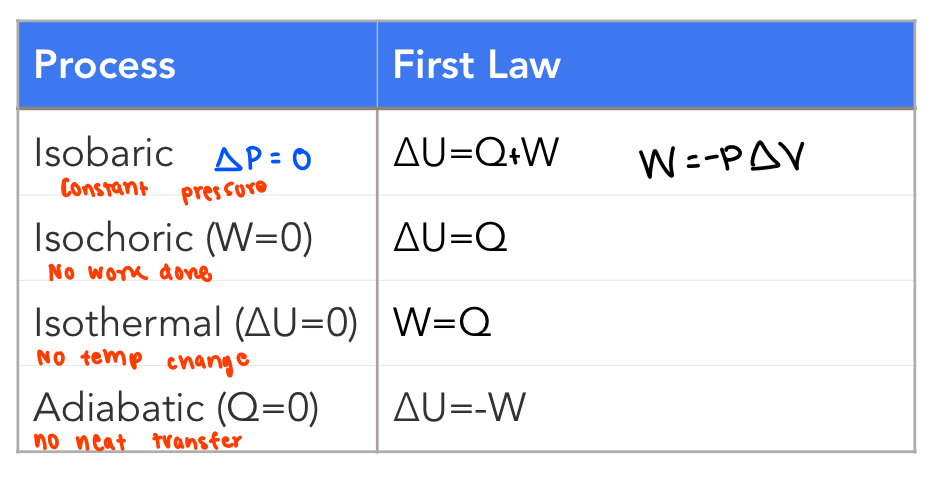
what is the second law of thermodynamics?
the total entropy of a system increases or remains constant in a spontaneous process → never decreases
what is entropy (S)?
a measure of how much energy is not available to do work; disorder
higher entropy= less energy available to do work
systems naturally evolve towards higher entropy states